Abstract
Platelet (PLT) production represents the final stage of megakaryocyte (MK) development. During differentiation, bone marrow MKs extend and release long, branched proPLTs into sinusoidal blood vessels, which undergo repeated abscissions to yield circulating PLTs. Circular-prePLTs are dynamic intermediate structures in this sequence that have the capacity to reversibly convert into barbell-proPLTs and may be related to “young PLTs” and “large PLTs” of both inherited and acquired macrothrombocytopenias. Conversion is regulated by the diameter and thickness of the peripheral microtubule coil, and PLTs are capable of enlarging in culture to generate barbell-proPLTs that divide to yield 2 smaller PLT products. Because PLT number and size are inversely proportional, this raises the question: do macrothrombocytopenias represent a failure in the intermediate stages of PLT production? This review aims to bring together and contextualize our current understanding of terminal PLT production against the backdrop of human macrothrombocytopenias to establish how “large PLTs” observed in both conditions are similar, how they are different, and what they can teach us about PLT formation. A better understanding of the cytoskeletal mechanisms that regulate PLT formation and determine PLT size offers the promise of improved therapies for clinical disorders of PLT production and an important source of PLTs for infusion.
Platelet production from bone marrow MKs
The proplatelet (proPLT) model of PLT formation recognizes that differentiated megakaryocytes (MKs) extend long, branching processes, designated proPLTs, which are composed of PLT-sized swellings in tandem arrays that are connected by thin cytoplasmic bridges.1 From as early as 1969, physiologic evidence of proPLT production has been supported by multiple images of proPLTs extending into the sinusoidal blood vessels of the bone marrow.2–4 Extracellular matrix (ECM) proteins are a major constituent of the bone marrow vascular niche and have been shown to promote proPLT formation in vitro.5,6 Nevertheless, how MKs establish the polarity needed to direct proPLT extension toward sinusoidal blood vessels and what effect ECM components have in regulating the rate, number, or size of their PLT products remain unclear. As proPLTs extend through the lumen of myeloid sinusoids, intravascular shear forces probably contribute to their elongation and release. In culture, proPLT-producing MKs release intermediate barbell structures that yield individual PLTs.1,7–9 These barbell structures have also been identified in the whole blood of rats10 and guinea pigs.11
In 2007, Junt et al used live imaging with multiphoton intravital microscopy to visualize PLT production in vivo.12 Although bone marrow MKs were shown extending proPLTs into sinusoidal blood vessels,13,14 the majority of shed fragments were larger than PLTs and confirmed that proPLTs were being released into the circulation. Originally proposed by Behnke and Forer in 1998,15 this study renewed support for the prediction that PLT morphogenesis continues in the vasculature, possibly assisted by intravascular shear forces. In the first half of this review, advances in the understanding of terminal PLT production are summarized individually under their respective title headings. In the second half of this review, common underlying connections deriving from these studies are examined collectively to establish a mechanistic model of PLT formation and size determination.
Terminal platelet production occurs in the bloodstream
Although the relative contributions of various organs to platelet production are still controversial, examination of whole blood in rats has revealed that proPLT production significantly increases after acute blood loss.10 In these studies, blood proPLT concentrations in right ventricles were significantly higher than in left ventricles of healthy animals. Likewise, reports of higher PLT counts in postpulmonary vessels suggest that the lung may be a milieu for terminal PLT formation.16 Indeed, the lung is the first capillary bed encountered by any cell leaving the bone marrow; and in humans, it has been reported that 10 times as many intact MKs are found in the pulmonary arterial blood than in blood collected from the aorta.17 Nevertheless, mice treated to perturb normal thrombopoiesis (platelet antiserum, 5-fluorouracil, and radioactive strontium) still contain significantly fewer MKs in the liver and lung than in the bone marrow or spleen (which were greatly increased over controls).18 Although this implies that the bone marrow and spleen are the major thrombopoietic organs in mice, proPLTs released from these sites may become trapped in the microcapillaries of the lung where intravascular shear forces can drive PLT production. Indeed, MKs infused into mice retro-orbitally or by tail vein mostly localize to the pulmonary vasculature, where they result in clinically relevant increases in PLT numbers over a period of 12 to 24 hours.19 Likewise, chloromethylfluorescein diacetate-labeled proPLTs are not detected in recipient mouse blood after infusion despite observations that chloromethylfluorescein diacetate-labeled PLT counts increase significantly over the same period of time.20 Identification and subsequent elaboration of microenvironments supporting thrombocytopoiesis will yield approaches to accelerate PLT production in vivo and establish bioreactors to replicate this process in culture.
The prePLT
In 2011, a new intermediate stage in the maturation process called the prePLT was discovered (Figure 1).20 PrePLTs were defined as anucleate PLT progenitors, 3 to 10 μm across, and may be related to “young (reticulated) PLTs” associated with increased RNA content, and “large PLTs” of inherited/acquired macrothrombocytopenias. Originally isolated from mouse MK cell cultures, we have recently identified circular-prePLTs in human whole blood.21 These dynamic intermediate structures reversibly convert into barbell-shaped proPLTs that divide to form 2 PLTs. Indeed, PLT distribution curves are known to be markedly asymmetric (with newly formed PLTs exhibiting larger dimensions than PLTs of a normal population),22 and the frequency distribution of PLT volume histograms is significantly changed in cases of severe PLT depletion.23 PrePLTs have yet to be isolated from MK cultures and whole blood apart from PLTs, and their functionality remains to be characterized.
Cytoskeletal model of PLT production. Top left: immunofluorescence microscopy images of a proPLT producing MK, released barbell-proPLTs, and circular-prePLTs from a mouse fetal liver cell culture probed for β1-tubulin; rapid-freeze electron microscopy image of the PLT cytoskeleton. Bottom left: list of inherited thrombocytopenias affecting platelet size, grouped by underlying defect; list of common causes of acquired thrombocytopenia. Top right: model of the terminal stages of PLT production. Released proPLTs undergo successive rounds of fission along their midbody and at their ends to generate circular prePLTs and barbell-proPLTs. Circular prePLTs reversibly convert into barbell-proPLTs, from which PLTs are released after a final fission event at their midsection. PLTs may enlarge during culture and contribute to further PLT production. Bottom right: model of suspected errors in terminal PLT production that can account for phenotypes expressed in common inherited and acquired thrombocytopenias. For immunofluorescence microscopy, samples were fixed with 4% formaldehyde for 15 minutes and then permeabilized with 0.5% Triton X-100 and blocked in immunofluorescene blocking buffer (1% BSA, 0.05% sodium azide, and 10% FCS in PBS) overnight before antibody labeling. To demarcate permeabilized cells, samples were incubated with a rabbit polyclonal primary antibody for mouse tubulin generated against the C-terminal peptide sequence LEDSEEDAEEAEVEAEDKDH (Genemed Synthesis). Samples were treated with a secondary goat anti–rabbit antibody conjugated to an Alexa Fluor 488 (Invitrogen). Samples were examined with an Axiovert 200 microscope (Carl Zeiss) equipped with a 63× (numeric aperture, 1.4) PlanApoChromat oil immersion objective, and images were obtained using a charged coupled device camera (Hamamatsu). Images were analyzed using Metamorph image analysis Version 7.7.2.0 software (Molecular Devices) and ImageJ Version 1.45r software. Professional illustration by Alice Y. Chen.
Cytoskeletal model of PLT production. Top left: immunofluorescence microscopy images of a proPLT producing MK, released barbell-proPLTs, and circular-prePLTs from a mouse fetal liver cell culture probed for β1-tubulin; rapid-freeze electron microscopy image of the PLT cytoskeleton. Bottom left: list of inherited thrombocytopenias affecting platelet size, grouped by underlying defect; list of common causes of acquired thrombocytopenia. Top right: model of the terminal stages of PLT production. Released proPLTs undergo successive rounds of fission along their midbody and at their ends to generate circular prePLTs and barbell-proPLTs. Circular prePLTs reversibly convert into barbell-proPLTs, from which PLTs are released after a final fission event at their midsection. PLTs may enlarge during culture and contribute to further PLT production. Bottom right: model of suspected errors in terminal PLT production that can account for phenotypes expressed in common inherited and acquired thrombocytopenias. For immunofluorescence microscopy, samples were fixed with 4% formaldehyde for 15 minutes and then permeabilized with 0.5% Triton X-100 and blocked in immunofluorescene blocking buffer (1% BSA, 0.05% sodium azide, and 10% FCS in PBS) overnight before antibody labeling. To demarcate permeabilized cells, samples were incubated with a rabbit polyclonal primary antibody for mouse tubulin generated against the C-terminal peptide sequence LEDSEEDAEEAEVEAEDKDH (Genemed Synthesis). Samples were treated with a secondary goat anti–rabbit antibody conjugated to an Alexa Fluor 488 (Invitrogen). Samples were examined with an Axiovert 200 microscope (Carl Zeiss) equipped with a 63× (numeric aperture, 1.4) PlanApoChromat oil immersion objective, and images were obtained using a charged coupled device camera (Hamamatsu). Images were analyzed using Metamorph image analysis Version 7.7.2.0 software (Molecular Devices) and ImageJ Version 1.45r software. Professional illustration by Alice Y. Chen.
The duplicating PLT
The discovery of a prePLT intermediate coincided with observations by Schwertz et al that anucleate human PLTs, maintained in suspension at 37°C, became larger and gave rise to new cell bodies packed with respiring mitochondria and α-granules.24 Because increasing PLT numbers were not generally observed during PLT unit storage, it has been proposed that PLTs might respond to decreases in local concentration by initiating duplication. Barbell-proPLT conversion in both human and mouse cultures was followed by the formation of a cleavage furrow along the long shaft between the 2 PLT-sized tips that may denote the point of division.20,24 Newly formed PLTs were structurally indistinguishable from blood PLTs, adhered and spread on ECM, and displayed normal signal-dependent expression of surface P-selectin and annexin V,24 suggesting that subpopulations of duplicating human PLTs may be analogous to prePLTs. Although a biomarker to distinguish between late-stage intermediates of PLT production and terminally differentiated PLTs is currently lacking, ongoing efforts to discover these will be paramount in resolving the mechanism of PLT duplication. Supporting this endeavor is the observation that human PLT progeny formation is accompanied by increases in protein synthesis and mitochondrial DNA replication, and translational inhibition with puromycin reduced the development of new cell bodies in these cultures.24 The observation that PLTs are capable of continued protein translation during culture25,26 has raised the question of whether the entire PLT proteome needs to be replicated to support PLT duplication or if translation of just a subset of this proteome is sufficient.
Acquired thrombocytopenias
Acquired thrombocytopenia is a major clinical problem encountered across a number of conditions, including immune (idiopathic) thrombocytopenic purpura, myelodysplastic syndromes, aplastic anemia, HIV infection, major cardiac surgery, chemotherapy, and radiation-induced thrombocytopenia (Figure 1). The latter represents the most common cause of low PLT counts in clinical hematology and oncology, and it has been estimated that 300 000 persons yearly worldwide undergo courses of chemotherapy adequate to produce clinically significant thrombocytopenia.27 Understanding the molecular basis of PLT size is also important because subjects with abnormally high PLT volumes have enhanced platelet reactivity and may be at risk for recurrent ischemic coronary syndromes.28 “Large PLTs” often occur concomitantly with periods of low PLT counts in humans,22 and increased PLT size may be the result of drug-induced effects on proPLT production and PLT release, or specific enlargement of circulating PLTs resulting from disruption of the cytoskeletal components regulating their peripheral microtubule coil.21 Alternatively, circulating prePLTs may represent newly released, young PLTs during rebound from PLT clearance. In addition to being larger, young PLTs are also more granulated and more reticulated (express higher concentrations of mRNA, mitochondrial DNA, and dense granules) than the majority of circulating PLTs in normal controls, which should set them apart from duplicating PLTs.29–34 Although the distinction between duplicating and young PLTs is important to make, the increased concentration of barbell-proPLT and circular-prePLT structures in stored PLT cultures and thrombocytopenic patient blood is not mutually exclusive and may represent a dynamic intermediate stage common to both.
Genetic regulators of PLT size
The fact that baseline peripheral blood cell parameters are influenced by genetics is firmly established, as heritability estimates derived from studies of PLT counts in twins have shown.35 An examination of the Mouse Phenome Database (http://www.jax.org/phenome) provides clear evidence of this in mice, with substantial variation observed in both PLT count and mean PLT volume (MPV) among 42 inbred strains, despite exposure to an identical environment.36 Although significant variability in PLT count and MPV has also been noted in humans, identifying their underlying genetic basis is often difficult because of environmental influences, genetic diversity, and small population size. Nevertheless, concordance between quantitative trait locus/loci (QTL) analyses in the mouse and human has been demonstrated for multiple complex traits and diseases.37–39 Genome-wide scans of QTL for PLT count and MPV in F2 intercrosses between NZW/LacJ, SM/J, and C57BLKS/J inbred mice revealed 3 significant QTL for baseline PLT count, and 2 significant QTL for MPV.36 Of these, 3 specific genes stand out: Tub2a (α2 tubulin), Tuba6 (α6 tubulin), and Nfe2 (nuclear factor, erythroid derived 2) encoding the 45-kDa subunit of the heterodimeric transcription factor NF-E2.40,41
Likewise, whole-blood measurements in 683 normal human subjects also reveal that there is a nonlinear, inverse relation between PLT count and MPV in humans,42 and imply that PLT number and size are fundamentally governed by a conservation of mass. This observation was most recently confirmed during a high-powered meta-analysis of genome-wide association studies in 66 876 persons of European ancestry.43 In this study, Gieger et al identified 68 genomic loci reliably associated with PLT count and volume mapping to established and putative novel regulators of megakaryopoiesis and PLT formation.43 Interestingly, many of the genes identified also correlated with known regulators of macrothrombocytopenia.
Inherited human macrothrombocytopenias
Among those genes most prominently expressed in the Gieger et al study were GPIbα, GPIX, and VWF, associated with Bernard-Soulier syndrome; GPIIb, associated with Glanzmann thrombasthenia; NF-E2; and β1-tubulin (Figure 2). One of the most distinguishing features of the resting PLT is its marginal microtubule coil.44 αβ-tubulin dimers polymerize into microtubules under physiologic conditions and are a key component of proPLT formation and PLT release.45,46 Likewise, the transcription factor p45 NF-E2, whose downstream targets include both the MK- and PLT-specific β1-tubulin, and Rab27b, is required for PLT formation (see “The microtubule cytoskeleton”).40,41,47–49 These data suggest that macrothrombocytopenias are in essence defects in terminal PLT production and may therefore offer direct insight as to the biophysical properties governing barbell-proPLT conversion and PLT release. The best characterized instances of human macrothrombocytopenias have been categorized by the cellular component affected and are described in the following sections.
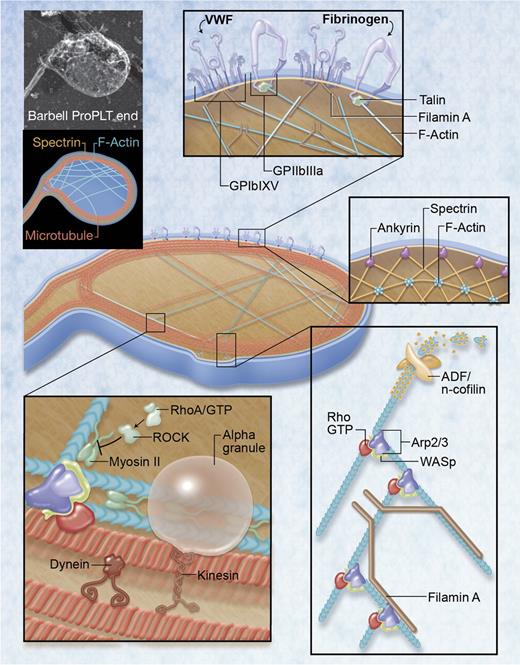
Regulatory factors governing PLT formation and size. Top left: rapid-freeze electron microscopy image and illustration of major cytoskeletal components in the barbell-proPLT end. Boxed regions highlight specific regulatory factors governing PLT formation and size. For rapid-freeze electron microscopy, cells were placed in a solution of 0.75% Triton X-100 in piperazine-N,N-bis-2-ethanesulfonic acid, N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, ethyleneglycoltetraacetic acid, and MgCl2 (PHEM) containing 0.1% glutaraldehyde, 5μM phalloidin, and 30μM taxol, and attached to the surface of poly-L-lysine–coated coverslips by centrifugation at 280g for 5 minutes. The cytoskeleton was rinsed in PHEM solution and fixed for 15 minutes in 1% glutaraldehyde in PHEM. Coverslips were washed in distilled water, rapidly frozen in a liquid helium–cooled copper block, transferred to a liquid nitrogen–cooled stage, freeze-dried at −90°C, and metal cast with 1.2 nm of tantalum-tungsten with rotation at 45° and 3 nm of carbon at 90° without rotation. Replicas were floated, picked up on formvar-carbon–coated grid, and examined in a JEOL 1200-EX transmission electron microscope at 80 kV. Professional illustration by Alice Y. Chen.
Regulatory factors governing PLT formation and size. Top left: rapid-freeze electron microscopy image and illustration of major cytoskeletal components in the barbell-proPLT end. Boxed regions highlight specific regulatory factors governing PLT formation and size. For rapid-freeze electron microscopy, cells were placed in a solution of 0.75% Triton X-100 in piperazine-N,N-bis-2-ethanesulfonic acid, N-2-hydroxyethylpiperazine-N′-2-ethanesulfonic acid, ethyleneglycoltetraacetic acid, and MgCl2 (PHEM) containing 0.1% glutaraldehyde, 5μM phalloidin, and 30μM taxol, and attached to the surface of poly-L-lysine–coated coverslips by centrifugation at 280g for 5 minutes. The cytoskeleton was rinsed in PHEM solution and fixed for 15 minutes in 1% glutaraldehyde in PHEM. Coverslips were washed in distilled water, rapidly frozen in a liquid helium–cooled copper block, transferred to a liquid nitrogen–cooled stage, freeze-dried at −90°C, and metal cast with 1.2 nm of tantalum-tungsten with rotation at 45° and 3 nm of carbon at 90° without rotation. Replicas were floated, picked up on formvar-carbon–coated grid, and examined in a JEOL 1200-EX transmission electron microscope at 80 kV. Professional illustration by Alice Y. Chen.
Glycoprotein expression
β3-integrin
Glanzmann thrombasthenia is caused by mutations in the ITGA2B or ITGB3 genes that encode for the integrin αIIbβ3 and results in a severe PLT bleeding disorder because of a limited ability to bind fibrinogen.50 Although PLT counts are typically normal in this disease, the recent discovery of autosomal dominant macrothrombocytopenias associated with PLT dysfunction has suggested that β3-integrin and its β-terminal domain are required for regulating normal proPLT formation and terminal PLT size.50,51 Although proPLT-extension in the p.D647_E686del mutation has yet to be examined, in vitro differentiated MKs with the β3-D723H mutation exhibit larger proPLT tips.37 This is characteristic of many human macrothrombocytopenias and suggests that the inability to further constrict proPLT ends before abscission may account for terminal PLT size.
T-box containing transcription factor 1
DiGeorge or velocardiofacial syndrome is thought to be caused by a haploid insufficiency of a single gene, TBX1 (encoding a T-box containing transcription factor)52 and can show autosomal recessive inheritance, although it is mostly acquired. Phenotypic features include conotruncal cardiac abnormalities, learning disabilities, velopharyngeal insufficiency, immunodeficiency, facial dysmorphism, and thymic hypoplasia.53 Although approximately 20% of patients express mild thrombocytopenia and increased PLT size, it should be noted that the GP1BB gene sits adjacent to TBX1, and its deletion can give rise to Bernard-Soulier syndrome in a compound heterozygote when accompanied by a pathologic mutation in the GATA-binding site of the remaining GPIbβ allele.54,55 Indeed, hemizygosity of GPIbβ in the 22q11.2 deletion syndrome is associated with macrothrombocytopenia in humans,55 as is targeted GPIBB disruption in mice,56 suggesting that haploinsufficiency of GPIBB in the right genetic background may be more related to PLT size and number than the absence of TBX1. Further work is therefore necessary to establish the significance of TBX1 in terminal PLT production.
Glycoprotein Ib
First described more than 60 years ago, Bernard-Soulier syndrome is an autosomal recessive macrothrombocytopenia linked to genetic lesions of the GPIb-IX-V complex that result in defective ristocetin-induced PLT agglutination, a lack of VWF-dependent PLT adhesion to subendothelium, and reduced thrombin-induced PLT aggregation.53,57 Normal numbers of MKs are generally found in the bone marrow of these patients, which implies that the macrothrombocytopenia is related to an error in the terminal stages of PLT production. MKs exhibit disordered and vacuolated invaginated membrane systems.57 Platelet counts in Bernard-Soulier syndrome mostly range from 40 000/μL to near normal and are also associated with abundant cytoplasmic vacuoles and an abnormal concentration of membrane complexes in discrete zones.53 GPIbα is most frequently affected in Bernard-Soulier syndrome, and GPIbα knockout mice recapitulate all the hallmarks of the Bernard-Soulier syndrome.57,58 In variant Bernard-Soulier syndrome, although nonfunctional GPIbα is partially expressed on the PLT surface, PLTs remain giant.53 Conversely, knocking out GPV in mice has no effect on MK ultrastructure and development of the invaginated membrane system, PLT production, size, adhesion, or GPIb-IX expression, and is consistent with the fact that no mutation in the GPV gene has yet been described in the Bernard-Soulier syndrome.59
The microtubule cytoskeleton
PLT shape and integrity are maintained by a well-defined and highly specialized cytoskeleton composed of an intricate system of molecular struts and girders that also regulate PLT size. The 3 major cytoskeletal components in PLTs are the marginal MT band, the actin-based cytoskeleton, and the spectrin-based membrane skeleton. Microtubules are composed of a lattice of αβ-tubulin heterodimers that form rigid tubular filaments.60 Microtubule-associated proteins crosslink and bundle microtubules, regulating their rigidity and stability, and actin and myosin attachments can create tensile force.60 During proPLT formation, MK extensions become filled with thick bundles of hundreds of microtubules that undergo a thinning phase down to approximately 20 microtubules. These microtubules loop within the ends of proPLTs and reenter the shaft, forming PLT-sized buds at the proPLT tip (Figure 1).61 ProPLT extension is the result of the continuous polymerization of tubulin bundles at their free plus ends, and dynein-powered sliding of overlapping microtubules.62 Because proPLT extension is a microtubule-driven process, force constraints resulting from cortical microtubule band organization and thickness should regulate PLT diameter by affecting flexural rigidity at the proPLT tip. MKs from Gray PLT syndrome, Bernard-Soulier syndrome, Filaminopathy A, May-Hegglin anomaly, and Epstein syndrome all have thicker peripheral microtubules and produce larger proPLT tips. Likewise, released PLTs contain 10- to 20-fold increased numbers of peripheral microtubule coils, relative to normal controls, that are often organized like balls of yarn rather than rings, which may explain why the majority of these cells have a spherical rather than the discoid form.21,63 The inability to further constrict proPLT tips before abscission resulting from bending energies determined by the cortical microtubule band could account for the increase in terminal PLT size.
β1-tubulin
Mature MKs, like PLTs, predominantly express the hematopoietic line-specific β1-tubulin isoform, which plays a specialized role in PLT synthesis, structure, and function. The localization of β1-tubulin to the proPLT marginal band is necessary for proPLT and PLT formation. Transgenic studies show that PLTs from mice lacking β1-tubulin are spherical and contain defective marginal bands with reduced microtubule coils (2 or 3 vs 8-12).64,65 These mice are also thrombocytopenic and express circulating PLT counts less than 50% of normal. A human β1-tubulin base pair substitution (AG → CC) resulting in a Q43P mutation has been identified and demonstrated to cause both functional and structural PLT alterations.66 The Q43P β1-tubulin variant was found in 10.6% of the general human population and in 24.2% of 33 unrelated patients with undefined congenital macrothrombocytopenia. Ultrastructural studies revealed large spherical PLTs with a disrupted microtubule coil and structural alterations.
The actin cytoskeleton
Little is known about the role of actin in proPLT production. Similar to tubulin, actin is in a dynamic monomer-polymer equilibrium. Actin filaments crosslink at various points to generate stiff cytoplasmic networks that intersect the microtubule cytoskeleton. Repeated actin-dependent bending and branching that bifurcates the proPLT shaft are common and serve to increase the number of free proPLT ends from which platelets are thought to release.61 Likewise, actin-modulating proteins, such as filamin, actin-depolymerizing factor (ADF)/n-cofilin, myosin, and WASp, affect tensile forces established during cytoskeletal remodeling and may regulate PLT size.
Filamin A
In PLTs, Filamin A anchors integrins β1, β3, and GPIb to the underlying actin cytoskeleton.61,67,68 Filamin A promotes high angle branching of actin filaments, organizing them into a 3-dimensional network that gives mechanical stability to the cell.69 Both Filamin A knockout and Filamin A/B knockdown mice have severe thrombocytopenia, decreased expression, and altered surface distribution of GPIbα, as well as PLT signaling and functional defects.69,70 ProPLT extensions in these MKs are composed of thicker microtubule bundles, contain more pronounced teardrop-shaped tips, and result in larger PLTs with thicker peripheral microtubule coils.71 Although mutations in the FLNA gene are responsible for a wide spectrum of rare diseases in humans, most studies to date have concentrated on FLNA-periventricular nodular heterotopias with seizures and cognitive defects.72 More recently, Nurden et al have reported a new subtype of Filaminopathy A in humans that was also associated with PLT macrothrombocytopenia.72 Although not all patients with FLNA mutations express macrothrombocytopenia, these data suggest that the disrupted linkage between the cytoplasmic tail of GPIbα and components of the PLT membrane cytoskeleton may form the molecular basis for resulting PLT size defects.
ADF/n-cofilin
Members of the ADF/cofilin family regulate actin turnover by associating preferentially with ADP-bound F-actin on dephosphorylation.73 This binding induces twisting of the actin filament, enhancing actin turnover by filament severing, and dissociation of actin monomers from the minus (pointed) end.74 Whereas ADF knockout mice have normal PLT counts, mice lacking n-cofilin display moderate thrombocytopenia and PLTs are markedly increased in size, suggesting that n-cofilin can functionally compensate for the loss of ADF in PLTs.74 Knocking out both ADF and n-cofilin severely limits proPLT extension, prevents formation of PLT-sized buds at proPLT tips, and virtually abolishes PLT release.74 Actin interacting protein 1, a cofilin-interacting protein, has been shown to enhance cofilin's capacity to sever actin filaments and may accelerate depolymerization by capping their barbed ends.75–78 Mutation in the gene encoding the mammalian homolog of Actin interacting protein 1, WD40-repeat protein 1, causes profound megakaryocytosis and macrothrombocytopenia in mice.79 Taken together, these data suggest that actin filament turnover plays a critical role in PLT production and the terminal stages of PLT release.
Myosin IIA
Myosin IIA, the nonmuscle myosin heavy chain product of the MYH9 gene, has also been implicated in restraining proPLT formation in developing MKs.80 Indeed, patients with autosomal dominant inherited MYH9-RDs (May-Hegglin anomaly, Fechtner syndrome, Sebastian PLT syndrome, and Epstein syndrome) generally exhibit macrothrombocytopenia as well as variable degrees of hearing loss, nephritis, and cataracts.81 MK maturation does not appear to be affected. Myosin IIA has ATPase activity and is responsible for actin-based motility in PLTs, monocytes, granulocytes, and other organs, such as the kidney, eye, and ear.72,82 Distinct mutations of Myosin IIA are associated with the severity of the hematologic phenotype in MYH9-RDs. Indeed, 79% of patients with an MYH9-RD carry mutations in only 6 of 1960 residues of Myosin IIA: Ser96 (6%) and Arg702 (24%) of the globular head; Arg1165 (9%), Asp 1424 (20%), and Glu 1841 (22%) of the coiled-coil domain; and Arg1933 (19%) of the nonhelical portion of the tail domain.83 Changes in the head domain variably affect the ability of Myosin IIA to hydrolyse ATP and slide along actin, whereas mutations in the tail domain alter to different degrees the conformation of the molecule and its ability to form filaments.83 In the case of MYH9-RD, defective Myosin IIA could promote premature proPLT formation in the osteoblastic niche through loss of attenuation or result in defective proPLT formation and reduced or abnormal PLT release in the vasculature.81
Conventional Myosin II complexes contain a dimer of heavy chains, each associated with the myosin light chain (MLC).80 MLC phosphorylation in MKs is regulated by RhoA-mediated activation of Rho-associated, coiled-coil containing protein kinase (ROCK). RhoA is central to actin by regulating stress fiber formation and actomyosin contractility84 and has also been implicated in cytokinesis, regulation of microtubule dynamics, and formation of focal adhesions.85,86 Overexpression of a constitutively active RhoA leads to decreased proPLT formation that can be rescued by simultaneous expression of a dominant inhibitory MLC form.80,87 Exogenous expression or constitutive phosphorylation of the MLC has a similar effect, demonstrating that Rho activation inhibits proPLT production through ROCK.80 Conversely, Myh9 knockout mouse embryonic stem cells differentiate into MKs that are fully capable of proPLT formation.80 Likewise, overexpression of a dominant negative form of RhoA or inhibition of ROCK leads to decreased MLC2 phosphorylation and increased proPLT formation.61 MK-specific RhoA gene deletion in mice revealed a marked increase in the number and ploidy of bone marrow MKs, a 25% increase in PLT volume, and 50% decrease in PLT count, confirming its involvement in terminal PLT production.88
Wiskott-Aldrich syndrome protein
Unlike the aforementioned instances of inherited “large PLT” disorders, Wiskott-Aldrich syndrome is an X-linked recessive disease characterized by eczema, increased susceptibility to infection, and moderate to severe microthrombocytopenia in humans.89 Hereditary X-linked thrombocytopenia is a milder form of this disease that lacks the immune problems.89 Premature termination or deletions in Wiskott-Aldrich syndrome protein (WASp) abrogate its expression and lead to severe disease. WASp has a large number of binding partners and can regulate actin polymerization through activation of the Arp2/3 complex.90 Arp2/3 binds to the sides of existing actin filaments and initiates new actin filament growth at 70° angles, forming branched actin networks.91 Indeed, Arp2/3 complex contribution to actin filament nucleation in PLTs requires free barbed ends generated by severing and uncapping preexisting actin filaments.92
Although peripheral blood PLTs from Wiskott-Aldrich syndrome and X-linked thrombocytopenia patients are significantly smaller than those from healthy human controls, CD34+ cells isolated from the blood and marrow of these patients exhibited normal MK differentiation and proPLT formation in culture, and released PLTs of normal size.93 Interestingly, mouse models of Wiskott-Aldrich syndrome also produce PLTs of normal size and exhibit only mild thrombocytopenia.94 MKs from WASp knockout mice, by comparison, demonstrate a marked abrogation of SDF-1– induced chemotactic migration from the collagen I–rich osteoblastic niche to the collagen IV–rich vascular niche, loss of normal α2β1-mediated inhibition of proPLT formation, and premature release of PLTs ectopically within the bone marrow space.94 MKs from WASp knockout mice were also completely deficient of actin-rich podosomes normally induced by GPVI and α2β1 signaling on contact with collagen I.94 Actin-rich podosomes were restricted to the ventral surface in mouse MK cell cultures, and suggest that WASp-induced podosome assembly may provide localized adhesive contacts that regulate proPLT extension in the presence of extracellular matrix components.
The spectrin-based membrane skeleton
The PLT membrane skeleton consists of elongated spectrin strands that interconnect through actin filament binding.95 This network is composed of both erythroid and nonerythroid spectrins that laminate the cytoplasmic surface of both the open-canalicular and plasma membrane systems.96 The invaginated membrane system of MKs is thought to supply membrane during proPLT formation and is also largely supported by a spectrin-based membrane skeleton.96 Expression of the spectrin tetramer-disrupting construct spα2N1 inhibits invaginated membrane system maturation and proPLT production in MKs, and rapidly destabilizes proPLT extensions in proPLT-producing MKs, causing blebbing and swelling.96 In permeabilized barbell-proPLTs, addition of spα2N1 converts these cells to spheres, demonstrating that membrane skeletal continuity maintains the elongated, prefission shape thought to regulate terminal PLT production.96 Mouse models of spectrin deficiencies have also been tied to abnormal PLT function, including stroke and thrombus formation.97,98 In humans, mutations in ANKRD26 have recently been associated with thrombocytopenia without macrocytosis.99 Ankyrin protein is responsible for the attachment of integral membrane proteins to the spectrin-actin based membrane skeleton and confirms that spectrin assembly is a critical factor in PLT formation.
Cytoskeletal model for PLT production
The production of PLTs by MKs requires an intricate series of cytoskeleton-driven remodeling events that result in the release of thousands of PLTs from a single progenitor. The formation of proPLTs and the final release step of PLTs have many things in common. Both involve elongation, dramatic membrane reorganization, and forces derived from the cytoskeleton. Because of pronounced similarities in morphogenesis, elucidation of the structural mechanics of proPLT formation probably provides insights into how terminal PLT size is established. Central to MK differentiation is the development of the invaginated membrane system, an expansive and interconnected membrane network of cisternae and tubules that are continuous with the plasma membrane, and function as a membrane reservoir during proPLT production. Erythroid and nonerythroid spectrins that laminate the cytoplasmic surface of the invaginated membrane system provide the skeletal structure necessary to support its formation.96 To assemble and release PLTs, MKs evaginate their membranes to form large pseudopodia that elongate, thin, bend, and branch into multiple proPLT projections with PLT-size swellings at their ends.
Early in the maturation process, before erosion of the cell body begins, the MK cytoplasm is replete with long individual arrays of microtubules that cluster around the nucleus and radiate toward the cell margins. As proPLT production initiates, microtubules consolidate into cortical bundles situated just beneath the membrane surface of the first pseudopodia extended. The developing proPLT shafts lengthen and taper as the microtubules that fill them undergo a thinning phase (to ∼ 20 microtubules), looping around the proPLT tip and reentering the shaft to form teardrop-shaped buds at their ends.61 Repeated actin-dependent bending and branching bifurcate the proPLT shaft and serve to increase the number of free proPLT ends.61 Branching initiates when a proPLT shaft is bent into a sharp kink, which eventually folds back on itself, forming a loop in the microtubule bundle. As this loop elongates, it forms a new proPLT shaft branching from the side of the original proPLT. Thickenings along the proPLT shaft, although superficially resembling PLT bodies, do not contain microtubule rings characteristic of the tips but are instead composed of parallel microtubule bundles that diverge for short distances and then rejoin.1 Although these swellings appear at regular intervals along proPLT extensions, they are not larger in MK models of human macrothrombocytopenia, and their purpose has yet to be resolved.
As proPLTs mature and are ultimately released, peripheral microtubule coils at the proPLT tips assume a uniform thickness of approximately 7 microtubules and diameter of approximately 2 μm, which is consistent with terminal PLT size. Released proPLTs retain the same dimensions as the branched extensions of proPLT-producing MKs, and it is easiest to consider their morphology apart from the larger MK body. In released proPLTs, the microtubule bundle is best regarded as a node-spring-loop (akin to an elastic band) that stretches as microtubules are slid apart and compresses as they are brought together (Figure 1). Because released proPLTs form barbells in culture with narrow corridors and teardrop-shaped ends instead of circles of increasing or decreasing diameters, these deformations must be constrained by external compressive pressures.
The actin-based cytoskeleton and spectrin-based membrane skeleton are attractive candidates for these compressive forces. Disorders in actin/myosin organization (as is the case for Filaminopathy A, ADF/n-cofilin knock outs, and MYH9 related disorders) result in increased proPLT end-sizes and PLT diameters. Likewise, megakaryocytic defects in the amino-terminal domain of highly expressed transmembrane receptors (as is the case for Glanzmann thrombasthenia, DiGeorge/Velocardiofacial syndrome, and Bernard-Soulier syndrome) disrupt connection of the plasma membrane to the actin cytoskeleton and exhibit disordered and vacuolated invaginated membrane systems in addition to producing larger proPLT end sizes and PLT diameters. Indeed, treatment of proPLTs with the actin-destabilizing drugs latrunculin and cytochalasin D increases the peripheral microtubule coil diameter of the proPLT tips, whereas addition of the actin-stabilizing drug jasplankinolide to proPLTs constricts it.21 The spectrin-based membrane skeleton, by comparison, provides structural integrity to preserve the long, tubular shape of proPLT projections without which they assume a swollen appearance and released barbell-proPLTs become sphericized.96
Mathematical modeling of the interplay between the expansion force generated by microtubule coil sliding and/or polymerization at the proPLT tip and the compressive force deriving from the actin-myosin-spectrin cortex have allowed us to address how terminal PLT size is established.21 This model predicts that final PLT size is determined by the balance between microtubule bundling and bending forces, such that when characteristic protein forces and microtubule rigidity are factored in, the predicted PLT radius is in the micron range. Biologic examples of human/murine macrothrombocytopenia have confirmed these predictions and shown that PLT size increases slowly as the microtubule number in the peripheral bundle cross-section grows. Because proPLT formation is governed by the same cytoskeletal mechanics, this accounts for larger diameters of proPLT ends in MK models of Bernard-Soulier syndrome, Filaminopathy A, and MYH9-RDs.21,63
ProPLTs are released by bone marrow MKs into sinusoidal blood vessels and fragment into individual PLTs while circulating in whole blood.12 Quantification of PLT production from released proPLTs in culture reveals that PLTs are generated at an increasing rate in time as large proPLTs undergo repeated abscissions along their midbodies.20 Barbell-shaped proPLTs represent the penultimate step in this continuum from which 2-individual PLTs are generated. During maturation, barbell-proPLTs revert to circular-prePLTs by untwisting their peripheral microtubule coil.20 Whole blood-derived prePLTs are cytoplasmically indistinguishable from young (reticulated) PLTs and contain comparable numbers of peripheral microtubules. The increased concentration of large PLTs in the circulation of both humans and mice in instances of increased PLT clearance, such as antibody-mediated thrombocytopenia, implies that prePLTs are a dynamic intermediate stage in PLT production. Given the low relative concentration of barbell-proPLTs (0.05%) versus circular-prePLTs (3.63%) in venous blood, it is probable that, once circular-prePLTs convert into barbell-proPLTs, abscission is rapid.21 Alternatively, prePLTs may become trapped in the microcapillaries of the bone marrow, lung, and/or spleen where intravascular shear forces can drive PLT production. Indeed, reports of higher PLT counts in postpulmonary vessels suggests that the lung may be a milieu for terminal PLT formation.16
Mathematical modeling of circular-prePLT-to-barbell-proPLT conversion based on the balance of microtubule bundling, elastic bending, and actin-spectrin-myosin cortex forces predicts that force constraints resulting from cortical microtubule band diameter and thickness determine barbell-proPLT formation and limit PLT size.21 Membrane skeletal continuity, by comparison, maintains the elongated, prefissile shape of the barbell-proPLT and may regulate terminal PLT production.96 By increasing their number of peripheral microtubules or disassembling their spectrin-based membrane skeleton, prePLTs are incapable of undergoing further barbell-proPLT conversion, resulting in terminal PLTs of a larger proportional size. In this regard, macrothrombocytopenias represent a failure to convert prePLTs to barbell-proPLTs. Because PLT production from MK progenitors is governed by a conservation of mass, as is demonstrated by the inverse relation between PLT count and MPV, large PLTs should coincide with lower PLT counts. Thrombocytopenia may be alleviated in part by increased megakaryopoiesis and can be exacerbated by PLT fragility and increased clearance, such as in Filaminopathy A. Precise quantification of PLT release from individual MKs apart from PLT clearance will further resolve the basis of thrombocytopenia in these syndromes.
Taken together, these data demonstrate that in proPLT-producing MK membrane receptors interact through microtubules, actin, and spectrin to provide both the static and dynamic framework necessary to regulate terminal PLT size. These features of living architecture are the same principles that govern tensegrity architecture and will require a paradigm shift in the way we frame questions of PLT production.85 It should not be surprising that the net balance of competing internal/external forces maintains cell shape during thrombocytopoiesis. Motor proteins, such as dynein, kinesin, and myosin, are known to regulate cellular architecture during proPLT formation. Nevertheless, these observations emphasize the need for biophysical approaches to model proPLT production where the mechanical properties of extracellular matrices, such as rigidity and patterning, and environmental influences, such as shear, are as important as ECM protein- and growth-factor-dependent signaling through specific membrane receptors.100–102
In conclusion, the transition from MK to PLT is a complex process. Although the basic mechanisms of proPLT extension have been investigated, elucidating the specific molecular controls and cellular pathways involved in PLT formation is an unfinished task. To fully understand the final stages of PLT production, several questions must be addressed. First, what are the individual molecules that power the conversion of prePLT intermediates into PLTs? Second, during this process, how does the marginal microtubule coil form inside the nascent PLT? Third, what molecules regulate the size of mature PLTs? And last, do humoral regulators of the final PLT release step exist? Further examination of genetic defects resulting in PLT disorders and the continued molecular and biochemical study of MKs as they transition into PLTs are needed before these questions can ultimately be answered.
Acknowledgments
The authors thank Elisabeth Battinelli and Kellie Machlus for revision of this manuscript. They also thank John Hartwig for guidance with electron microscopy.
This work was supported in part by the National Institutes of Health (grant Hl68130, J.E.I.). J.E.I. is an American Society of Hematology Junior Faculty Scholar. J.N.T. is an American Society of Hematology Scholar.
National Institutes of Health
Authorship
Contribution: J.N.T. and J.E.I. designed and wrote the review.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Joseph E. Italiano Jr, Hematology Division, Department of Medicine, Brigham and Women's Hospital, 1 Blackfan Circle, Karp 6, Boston, MA 02115; e-mail: jitaliano@rics.bwh.harvard.edu.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal