To the editor:
The JAK1/JAK2 inhibitor ruxolitinib was recently approved for intermediate and high-risk myelofibrosis. However, for malignancies with JAK2 rearrangements, known for their rapid evolution and dismal prognosis, the potential of JAK inhibitor therapy is not established.1 We investigated the efficacy of ruxolitinib against JAK2 fusions in vitro and in a patient with t(8;9)(p22;p24)/PCM1-JAK2 chronic eosinophilic leukemia (CEL).
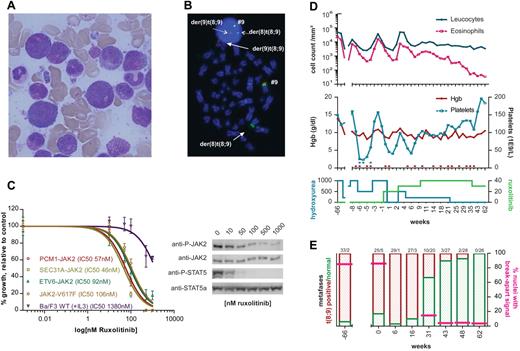
A 72-year-old male presented with leukocytosis of 49 × 109/L, absolute eosinophil count of 23 × 109/L and circulating myeloid progenitors. Specific symptoms were absent and clinical examination was unremarkable. Bone marrow aspirate and biopsy were hypercellular with prominent myelopoiesis, pronounced eosinophilia, and hypergranulated granulocyte precursors (Figure 1A). Cytogenetics showed 46,XY,t(8;9)(p22;p24) in 33 of 35 metaphases, and FISH confirmed the breakpoints in 8p22 (PCM1) and 9p24 (JAK2; Figure 1B). RT-PCR and Sanger sequencing showed an in-frame fusion between PCM1 exon 36 and JAK2 exon 9, as in the first case reported.2
Ruxolitinib inhibits PCM1-JAK2 in vitro and in vivo. (A) Bone marrow smear at diagnosis, showing hypereosinophilia and marked hypergranularity of neutrophils and their precursors. (B) FISH on a cytogenetic marrow specimen at diagnosis. Dual color JAK2 break-apart FISH was performed using the following probes: RP11 307I14–RP11 125K10–RP11 509D8 (Spectrum Green) and RP11 140C18–RP11 635N21 (Spectrum Orange), as described.5 The t(8;9) leads to translocation of the green signal to der(8), while the red probes and a tiny little green signal are retained on the der(9) (solid arrows). The same break-apart pattern is observed in interphase cells (dotted arrows). (C) Dose-response curves of Ba/F3 cells expressing PCM1-JAK2, ETV6-JAK2, SEC31A-JAK2, or JAK2-V617F (left panel). Cells were treated for 24 hours with various concentrations of ruxolitinib, diluted in DMSO immediately before use. The proliferation relative to untreated controls is shown. The growth of wild type Ba/F3 in the presence of IL-3, and various concentrations of ruxolitinib is also shown (curves fitted with GraphPad Prism 5). Western blot analysis of 2 × 106 PCM1-JAK2–transformed Ba/F3 cells after treatment with ruxolitinib for 90 minutes (right panel). Gel electrophoresis was performed using NuPage Bis-Tris 4%-12% gels (Invitrogen). Immunoblotting was done with anti-phospho-JAK2, anti-JAK2, anti-phospho-STAT5, anti-STAT5a (Cell Signaling Technology), and anti–mouse/anti–rabbit peroxidase-labeled antibodies (Amersham Biosciences). (D) Evolution of peripheral blood counts before and after initiation of ruxolitinib. The top panel depicts the evolution of peripheral leukocyte count (blue) and absolute eosinophil count (pink) over time. The middle panel shows hemoglobin levels (red) and platelet count (blue) over time. Erythrocyte or platelet transfusions are shown as red and blue dots above the x-axis. The average daily dose (mg) of hydroxyurea and ruxolitinib are shown in the bottom panel. Start of ruxolitinib treatment is time point 0. (E) Evolution of bone marrow karyotype and FISH. Bars show the relative proportion of t(8;9)-positive metaphases (red) versus normal metaphases (green). The number of t(8;9)-positive metaphases versus normal metaphases is indicated above each bar. Pink lines indicate the percentage of interphase nuclei with a break-apart pattern in these marrow specimens.
Ruxolitinib inhibits PCM1-JAK2 in vitro and in vivo. (A) Bone marrow smear at diagnosis, showing hypereosinophilia and marked hypergranularity of neutrophils and their precursors. (B) FISH on a cytogenetic marrow specimen at diagnosis. Dual color JAK2 break-apart FISH was performed using the following probes: RP11 307I14–RP11 125K10–RP11 509D8 (Spectrum Green) and RP11 140C18–RP11 635N21 (Spectrum Orange), as described.5 The t(8;9) leads to translocation of the green signal to der(8), while the red probes and a tiny little green signal are retained on the der(9) (solid arrows). The same break-apart pattern is observed in interphase cells (dotted arrows). (C) Dose-response curves of Ba/F3 cells expressing PCM1-JAK2, ETV6-JAK2, SEC31A-JAK2, or JAK2-V617F (left panel). Cells were treated for 24 hours with various concentrations of ruxolitinib, diluted in DMSO immediately before use. The proliferation relative to untreated controls is shown. The growth of wild type Ba/F3 in the presence of IL-3, and various concentrations of ruxolitinib is also shown (curves fitted with GraphPad Prism 5). Western blot analysis of 2 × 106 PCM1-JAK2–transformed Ba/F3 cells after treatment with ruxolitinib for 90 minutes (right panel). Gel electrophoresis was performed using NuPage Bis-Tris 4%-12% gels (Invitrogen). Immunoblotting was done with anti-phospho-JAK2, anti-JAK2, anti-phospho-STAT5, anti-STAT5a (Cell Signaling Technology), and anti–mouse/anti–rabbit peroxidase-labeled antibodies (Amersham Biosciences). (D) Evolution of peripheral blood counts before and after initiation of ruxolitinib. The top panel depicts the evolution of peripheral leukocyte count (blue) and absolute eosinophil count (pink) over time. The middle panel shows hemoglobin levels (red) and platelet count (blue) over time. Erythrocyte or platelet transfusions are shown as red and blue dots above the x-axis. The average daily dose (mg) of hydroxyurea and ruxolitinib are shown in the bottom panel. Start of ruxolitinib treatment is time point 0. (E) Evolution of bone marrow karyotype and FISH. Bars show the relative proportion of t(8;9)-positive metaphases (red) versus normal metaphases (green). The number of t(8;9)-positive metaphases versus normal metaphases is indicated above each bar. Pink lines indicate the percentage of interphase nuclei with a break-apart pattern in these marrow specimens.
This PCM1-JAK2 fusion was cloned and additional ETV6-JAK2 and SEC31A-JAK2 constructs were generated. In Ba/F3 cells, PCM1-JAK2, ETV6-JAK2, and SEC31A-JAK2 induced growth-factor independent growth,3-5 which was suppressed by ruxolitinib with IC50 values of 57, 46, and 92nM, respectively (Figure 1C). Western blotting showed dose-dependent inhibition of phosphorylation of JAK2, indicating a direct and specific action of ruxolitinib, and of downstream STAT5 (Figure 1C). For comparison, the growth of JAK2-V617F–expressing Ba/F3 was inhibited with 106nM as IC50, in line with published data.5,6 Similar data were obtained for JAK inhibitor I, a JAK1/JAK2/JAK3/TYK2 inhibitor (not shown).
Given this, and lacking effective medical treatment options, ruxolitinib was initiated after approval from the ethical committee and patient informed consent. Since diagnosis, the leukocyte count had been controlled with hydroxyurea 250-2000 mg/d, but the peripheral eosinophilia remained elevated. Multiple transfusions and dose adjustments were required for anemia, with corresponding fluctuations in leucocytes and eosinophils. After 15 months, ruxolitinib was started at 10 mg twice daily. At this point, 25 of 30 marrow metaphases were t(8;9)-positive (Figure 1D-E). Ruxolitinib was cautiously increased to 15-20 mg bid and hydroxyurea could be discontinued 6 months later. By then, the eosinophil count had normalized, and the thrombocytopenia partially recovered. Moreover, the number of t(8;9) metaphases had decreased to 10 of 30. Continuing ruxolitinib as single agent, the eosinophil count remained normal. Marrow examinations after 11 and 15 months ruxolitinib therapy were hypocellular without evidence of CEL. After 15 months, a complete cytogenetic response (0 abnormal of 26 analyzed metaphases) was reached; and the number of interphase nuclei with a JAK2/9p24 breakpoint in FISH had equally decreased to less than 5%. Apart from anemia, and increased erythrocyte transfusion need, the drug was excellently tolerated, without nonhematologic toxicities. Despite transient thrombocytopenia at ruxolitinib initiation, platelet transfusions were not required at any time later (Figure 1D-E).
In summary, the response after 62 weeks, as by cytogenetics/FISH, reflects a 1-2 log reduction in leukemic burden, delivering proof-of-principle that ruxolitinib directly suppresses the malignant clone. Yet, only 21% reduction of the JAK2-V617F burden was obtained in the COMFORT trials,7,8 although ruxolitinib's IC50 for PCM1-JAK2 is within the same order of magnitude as for JAK2-V617F. Thus, PCM1-JAK2–transformed cells are seemingly more dependent on JAK2 signaling than JAK2-V617F–positive progenitors. An important implication is that ruxolitinib may modify the natural history of PCM1-JAK2–positive disease, as imatinib does in CML and FIP1L1-PDGFRA–positive CEL. More clinical experience with ruxolitinib in PCM1-JAK2–positive myeloproliferative neoplasms is eagerly awaited.
Authorship
Acknowledgments: The authors would like to thank the technicians of the Center for Human Genetics for outstanding cytogenetic and FISH experiments, Dr L. Knoops (Cliniques Universitaires Saint-Luc, Université Catholique de Louvain and Ludwig Institute for Cancer Research, Brussels) for discussions, and Katrien Van Roosbroeck (KU Leuven, Leuven) for providing SEC31A cDNA. They also thank Novartis (Vilvoorde, Belgium) for kindly supplying ruxolitinib. This work was supported by grants from KU Leuven (GOA/11/010 to P.V.), FWO-Vlaanderen (G.0509.10 to P.V.), the Foundation against Cancer (grant 2010-204 to P.V. and E.L.), and vzw Wetenschappelijk Fonds Haematologie AZ Sint-Jan Brugge. E.L. is a postdoctoral fellow from FWO-Vlaanderen. P.V. is a senior clinical investigator from FWO-Vlaanderen.
Contribution: E.L. designed the in vitro study, performed research, analyzed the data, and wrote the paper; D.S. and J.B. treated the patient, provided patient data, and revised the article for intellectual content; S.S. performed research and analyzed the data; and P.V. performed cytogenetic and FISH analysis, designed the study, analyzed the data, and wrote the paper.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Peter Vandenberghe, Center for Human Genetics, University Hospital Leuven, Herestraat 49, B-3000 Leuven, Belgium; e-mail: peter.vandenberghe@uzleuven.be.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal