Despite the introduction of tyrosine kinase inhibitor therapy, the prognosis for p190-BCR-ABL+ acute lymphoblastic leukemia remains poor. In the present study, we present the cellular and molecular roles of the Rho GTPase guanine nucleotide exchange factor Vav in lymphoid leukemogenesis and explore the roles of Vav proteins in BCR-ABL–dependent signaling. We show that genetic deficiency of the guanine nucleotide exchange factor Vav3 delays leukemogenesis by p190-BCR-ABL and phenocopies the effect of Rac2 deficiency, a downstream effector of Vav3. Compensatory up-regulation of expression and activation of Vav3 in Vav1/Vav2–deficient B-cell progenitors increases the transformation ability of p190-BCR-ABL. Vav3 deficiency induces apoptosis of murine and human leukemic lymphoid progenitors, decreases the activation of Rho GTPase family members and p21-activated kinase, and is associated with increased Bad phosphorylation and up-regulation of Bax, Bak, and Bik. Finally, Vav3 activation only partly depends on ABL TK activity, and Vav3 deficiency collaborates with tyrosine kinase inhibitors to inhibit CrkL activation and impair leukemogenesis in vitro and in vivo. We conclude that Vav3 represents a novel specific molecular leukemic effector for multitarget therapy in p190-BCR-ABL–expressng acute lymphoblastic leukemia.
Introduction
Philadelphia chromosome–positive (Ph+) hematologic malignancies arise from the t(9,22) (q34;q11.2) mutation, which encodes the constitutively active tyrosine kinase oncofusion protein BCR-ABL. BCR-ABL is both necessary and sufficient to induce leukemia.1 Two types of BCR-ABL fusion proteins, associated with different break points in the BCR gene, have been identified in patients with Ph+ B-cell acute lymphoblastic leukemia (Ph+ B-ALL). A 190-kDa fusion protein, referred to as p190-BCR-ABL, is present in 60%-80% of Ph+ B-ALL cases. Leukemia induced by this BCR-ABL fusion protein arises from a transformed B-cell progenitor.2 A BCR-ABL isoform of 210 kDa, known as p210-BCR-ABL, is commonly expressed in patients with chronic myelogenous leukemia (CML) and in a minority of patients with Ph+ B-ALL. The transforming effect of BCR-ABL is dependent on the tyrosine kinase (TK) activity of the fusion protein that leads to autophosphorylation, recruitment of adaptor proteins, and subsequent activation of downstream signaling. The TK inhibitor (TKI) imatinib and the second-generation TKIs dasatinib and nilotinib have been used as frontline treatment for CML and Ph+ B-ALL patients.3 However, relapse is common in Ph+ B-ALL despite high rates of complete response with initial therapy,4,5 probably because of survival of leukemic progenitors. These BCR-ABL+ progenitors appear to accumulate additional genetic mutations that result in a proliferative advantage and differentiation arrest.6 Understanding the downstream signaling cascades activated by BCR-ABL may lead to the development of more effective therapeutic strategies that aim to prevent the development and/or selection of TKI-resistant clones.
Expression of p210-BCR-ABL activates the Rho-family GTPases Rac, RhoA, and Cdc42,7 possibly through the double homology (DH) domain of guanine exchange factors (GEFs).8 We demonstrated previously that the absence of Rac proteins, specifically Rac2 or the combination of Rac1 and Rac2, impairs myeloid leukemogenesis induced by p210-BCR-ABL expression in the hematopoietic stem and progenitor cell compartment.9,10 Activation of Rac GTPases, particularly Rac2, has been shown to regulate reactive oxygen species production by NADPH oxidase complexes11 and possibly to be responsible for DNA damage and genetic instability in BCR-ABL leukemias.12 Expression of p190-BCR-ABL also activates Rac GTPases7 despite a lack of the DH domain, suggesting that the activation of Rho-family GTPases by p190-BCR-ABL must depend on the expression and activation of alternative GEFs.
Vav proteins are GEFs for Rho-family GTPase members.13 The mammalian Vav family is made up of 3 members: Vav1, Vav2, and Vav3. Despite common functional domains and similar mechanism of phosphorylation-dependent activation,14 the sequence homology between the 3 Vav isoforms is only approximately 65%. In addition, Vav1 expression is restricted to hematopoietic cells, whereas Vav2 and Vav3 are expressed broadly in multiple tissues.15 Overexpression studies and various Vav gene knockout mice have revealed both unique and redundant roles of the 3 Vav family members in lymphoid cells.16 The phosphorylation of Vav proteins on specific tyrosine residues leads to conformational changes required for binding to GTPase effectors.14 Vav1 has been shown to exist as a complex with both p190- and p210-BCR-ABL,17 with uncertain significance. Whether other Vav proteins complex BCR-ABL is not known.
In the present study, we explored the upstream mechanism of p190-BCR-ABL–dependent Rac activation through the Vav GEF family members. We show that, although both Vav1 and Vav3 are hyperactivated in primary human and murine p190-BCR-ABL+ B-ALL, Vav3-deficient leukemogenesis induced by p190-BCR-ABL is delayed. The proliferation and survival of B-cell progenitors is impaired by genetic loss of Vav3 and can be reverted by its reintroduction, and compensatory Vav3 up-regulation induced by genetic combined deficiency of Vav1 and Vav2 translates into increased survival and expansion ex vivo. Vav3 deficiency reduces p190-BCR-ABL–induced Rac GTPase activation and decreases proliferation and Vav3-deficient p190-BCR-ABL+ murine and human B-cell progenitors have enhanced apoptosis associated with augmented expression of Bad and Bik and the downstream effectors Bax and Bak. Our results demonstrate that Vav3 is a critical GEF in p190-BCR-ABL–mediated activation of Rac GTPase and down-regulation of proapoptotic signals required for leukemogenesis. We also demonstrate that Vav3 deficiency collaborates with TKI in the inhibition of leukemogenesis in vivo. Therefore, the results of the present study suggest that Vav3 may be a useful therapeutic target in p190-BCR-ABL+ B-ALL.
Methods
Animals
Vav-deficient mice16 and Rac2-deficient mice18 have been described previously. Vav1−/−;Vav2−/− mice were generated by intercrossing. All mutant mice had been backcrossed > 10 generations into C57Bl/10 or C57Bl/6 mice. Six- to 8-week-old female wild-type (WT) C57Bl/6 and C57Bl/10 mice were obtained commercially (The Jackson Laboratory and Harlan Laboratories) and used as donors and/or recipients of transduction/transplantation models. The Cincinnati Children's Hospital Medical Center institutional animal care and use committee approved the protocol.
Human specimens
Umbilical cord blood (UCB) cells, normal BM, and B-ALL low-density bone marrow (LDBM; p190-BCR-ABL+ or TEL-AML1+) specimens were obtained through institutional review board-approved protocols and donor informed consent from Cincinnati Children's Hospital Medical Center, Universidad de Navarra (Spain), or Shanghai Children's Medical Center (China). All leukemic specimens contained a minimum of 80% blasts and corresponded to specimens obtained at diagnosis or relapse.
Viral transduction
The retroviral vectors and generation methods have been used and described previously.9 In brief, Ba/F3 or mouse LDBM were transduced with bicistronic retroviral vectors (MIEG3)19 encoding BCR-ABL isoforms p190 or p210 in the presence of 20 ng/mL of recombinant human IL-7 (PeproTech), 10 ng/mL of recombinant mouse SCF (PeproTech), and the recombinant fragment of fibronectin, CH296 (Takara Bio) for 16 hours at 37°C. For the Vav3 rescue experiment, lentiviral particles encoding either WT Vav3 (full-length) cloned in a pCDH1-MCS1-EF1-copGFP vector (System Biosciences), or the empty vector, were used for stable expression in p190-BCR-ABL+ B-cell lymphoid progenitors. Retroviral transduction of human UCB CD34+ cells was performed under conditions similar to those used for murine cell transduction, but using recombinant human SCF instead. Lentiviral transduction of UCB CD34+ cells was performed using 2 rounds of infection with Vav3 shRNA-containing pLKO.1-puro-CMV-tGFP vectors (Sigma-Aldrich) at a multiplicity of infection of 20.
Transplantation of transduced leukemic cells
LDBM cells from WT or gene-targeted mice lacking Vav or Rac were isolated and transduced with either a retroviral bicistronic vector encoding p190-BCR-ABL with enhanced green fluorescent protein (EGFP) as a reporter or an empty vector solely expressing the reporter protein (Mock). Transduced LDBM cells (1-3 × 106) were intravenously transplanted into lethally (7 + 4.75 Gy) irradiated C57Bl/6 or C57Bl/10 mice. The multiplicity of infection was kept low to allow 1 single copy of BCR-ABL per transduced cell. Transduction efficiency of C57Bl/6 LDBM cells was not significantly different between groups (3.3% ± 1.8%, 2.3% ± 1.1%, and 5.1% ± 2.3% for WT, Rac2−/−, and Rac3−/− BM cells, respectively). Transduction efficiencies of C57Bl/10 LDBM cells were not significantly different among the groups (10.66% ± 2.12%, 10.86% ± 1.63%, and 11.5% ± 1.2% for WT, Vav3−/−, and Vav1−/−;Vav2−/− LDBM cells, respectively). Secondary recipients received the same irradiation dose and a cell dose of 4 × 105 sorted EGFP+ B220+cKit+ BM cells along with 3 × 106 normal, congenic C57Bl/10 BM cells. Peripheral blood was retro-orbitally collected on day 21 and on subsequent days after transplantation for complete blood counts and leukocyte differentials to check the engraftment of donor cells. Experimental mice were sacrificed when they showed signs of extreme hunched posture with labored breathing and/or symptoms of moribund or rear paralysis. BM and spleens were homogenized and processed for flow cytometric analysis. The remainder of cells were frozen for in vitro analysis.
CFU-proB assay
B-cell lineage colony-forming units (CFU-proB) were quantified after 9 days of culture of leukemic BM cells or sorted p190 BCR-ABL–expressing B-cell progenitors in M3134 methylcellulose (StemCell Technologies) supplemented with 30% FBS (for mouse B lymphoid colony forming cells; StemCell Technologies), 2mM l-glutamine (Invitrogen), 1% antibiotics (penicillin-streptomycin; Invitrogen), 100μM β-mercaptoethanol (Fisher-Scientific), 1% BSA (Sigma-Aldrich), 20 ng/mL of recombinant mouse IL-7 (PeproTech), and 100 ng/mL of recombinant mouse SCF (PeproTech).
For details on other methods, please see supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
Results
Vav3 is activated in p190-BCR-ABL+ leukemia
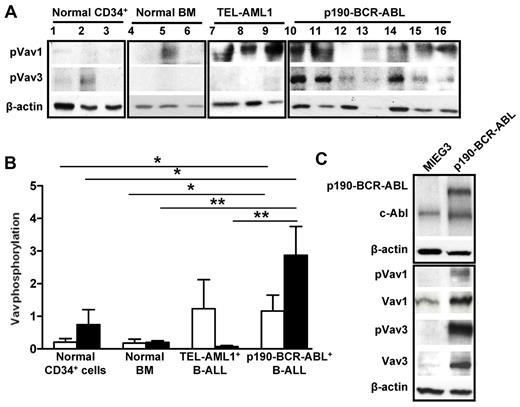
Vav mediates the activity of protein TKs, including BCR-ABL,20 as a result of the phosphorylation of a specific tyrosine residue in the Vav DH domain. Vav1 has been shown to be activated in BCR-ABL–expressing leukemias,17 but the level of activation of Vav3 in BCR-ABL leukemias is unknown. In the present study, we analyzed the level of Vav3 activation in human p190-BCR-ABL+ B-lymphoblastic leukemias. All 7 LDBM samples from p190-BCR-ABL+ B-ALL patients showed markedly increased levels of expression and phosphorylation of Vav1 and Vav3 compared with LDBM cells (7- and 14.3-fold increase, P < .05 and P < .01, respectively) and CD34+ cells (6.3- and 3.9-fold, respectively, P < .05) from normal controls (Figure 1A-B). In contrast, patients with TEL-AML1+ B-ALL BM cells (n = 3) showed normal activation of Vav1 and minimal activation of Vav3 compared with p190-BCR-ABL B-ALL cells (41-fold difference, P < .01; Figure 1A-B).
Vav1 and Vav3 are overexpressed and overactivated in p190-BCR-ABL ALL. (A) Representative immunoblot of phospho-Vav1 and phospho-Vav3 in human cells. Lanes 1 through 3 are CD34+ cells from healthy donors; lanes 4 through 6 are LDBM cells from healthy donors; lanes 7-9 are LDBM cells from patients with TEL-AML1+ B-ALL; and lanes 10 through 16 are LDBM cells from 7 patients with p190-BCR-ABL+ B-ALL. β-actin expression analysis was used as a loading control. Two independent experiments were run with the same specimens. (B) Normalized phosphorylation of Vav1 (empty bar) and Vav3 (solid bar) from the samples in panel A. The ratio was generated by dividing the densitometric value of each phosphorylated protein by that of β-actin from the same sample. Values represent average ± SEM. *P < .05; **P < .01. (C) Immunoblot of phosphorylated forms (Vav1-pTyr174 and Vav3-pTyr173) compared with total Vav1 and Vav3 in sorted murine B-220+/c-kit+/CD43+/EGFP+ B-cell progenitors transduced with either an empty vector (MIEG3) or p190-BCR-ABL.
Vav1 and Vav3 are overexpressed and overactivated in p190-BCR-ABL ALL. (A) Representative immunoblot of phospho-Vav1 and phospho-Vav3 in human cells. Lanes 1 through 3 are CD34+ cells from healthy donors; lanes 4 through 6 are LDBM cells from healthy donors; lanes 7-9 are LDBM cells from patients with TEL-AML1+ B-ALL; and lanes 10 through 16 are LDBM cells from 7 patients with p190-BCR-ABL+ B-ALL. β-actin expression analysis was used as a loading control. Two independent experiments were run with the same specimens. (B) Normalized phosphorylation of Vav1 (empty bar) and Vav3 (solid bar) from the samples in panel A. The ratio was generated by dividing the densitometric value of each phosphorylated protein by that of β-actin from the same sample. Values represent average ± SEM. *P < .05; **P < .01. (C) Immunoblot of phosphorylated forms (Vav1-pTyr174 and Vav3-pTyr173) compared with total Vav1 and Vav3 in sorted murine B-220+/c-kit+/CD43+/EGFP+ B-cell progenitors transduced with either an empty vector (MIEG3) or p190-BCR-ABL.
We hypothesized that Vav3 activation plays an important and nonredundant role in p190-BCR-ABL–mediated leukemogenesis. To test this hypothesis, we used Vav gene-knockout mice in which we transduced p190-BCR-ABL in LDBM cells. We first determined the leukemogenic effect of p190-BCR-ABL expression in primary B-cell progenitors. LDBM cells from specific gene-deleted murine models or WT mice were transduced with bicistronic vectors expressing EGFP and p190-BCR-ABL (MSCV-p190-BCR-ABL) or only EGFP (MIEG3), then cultured with IL-7 and SCF.21 The expression and activation of Vav family members were analyzed by quantitative RT-PCR and phospho-immunoblot in FACS-sorted B-220+/c-kit+/CD43+/EGFP+ leukemic cells from WT and Vav-deficient animals. Vav1 and Vav3, but not Vav2 mRNA, were detected in p190-BCR-ABL+ B-lymphoid progenitors (supplemental Figure 1A-C). Expression of p190-BCR-ABL in murine B-cell progenitors induced increased expression and activation of Vav1 and Vav3 (Figure 1C). We next examined the effect of expression of p190-BCR-ABL in Vav3-deficient mice. In these mice, p190-BCR-ABL expression in Vav3−/− B-cell progenitors was associated with up-regulation of both Vav1 and Vav2 mRNA expression (supplemental Figure 1A-B), suggesting the existence of compensatory mechanisms controlling the expression of Vav proteins.
Vav3 is uniquely required for leukemia cell proliferation and survival induced by p190-BCR-ABL, but not by p210-BCR-ABL
To assess the effects of Vav proteins on p190-BCR-ABL leukemogenesis, we analyzed whether the deficiency of these proteins modulates the proliferation and/or survival of p190-BCR-ABL+ B-cell progenitors. Deficiency of Vav3 impaired the expansion of p190-BCR-ABL+ CFU-proB cells, whereas, paradoxically, the combined deficiency of Vav1 and Vav2 increased the cumulative expansion of B-cell progenitors (Figure 2A). Nontransformed B-lymphoid progenitors showed limited expansion, which was independent of their genotype (Figure 2A).
Loss of Vav3 impairs leukemic proliferation and survival of p190-BCR-ABL+ B-cell progenitors. (A) Expansion (fold) of in vitro–cultured B-cell progenitors (CFU-proB) from WT (red lines), Vav1−/−;Vav2−/− (green lines), and Vav3−/− (blue lines) cells transduced with either p190-BCR-ABL (solid lines) or mock vector (MIEG3, dotted lines). (B) S-phase fraction as assessed by bromodeoxyuridine incorporation of B-cell progenitors expressing either mock vector (hatched bars) or p190-BCR-ABL (solid bars) on day 6 of in vitro culture. (C) Apoptosis as assessed by annexin V binding of leukemic p190-BCR-ABL+ B-cell progenitors on day 16 of in vitro culture. (D) Representative example of immunoprecipitation and Western blot of pTyr-Vav and total Vav3 in p190-BCR-ABL+ B-cell progenitors from WT, Vav1−/−;Vav2−/−, and Vav3−/− mice. Lysates from 5 × 105 B-cell progenitors were immunoprecipitated, loaded, and blotted with Abs against phospho-tyrosine and Vav3. β-actin expression analysis from total lysate was used as a loading control (n = 3 independent experiments).
Loss of Vav3 impairs leukemic proliferation and survival of p190-BCR-ABL+ B-cell progenitors. (A) Expansion (fold) of in vitro–cultured B-cell progenitors (CFU-proB) from WT (red lines), Vav1−/−;Vav2−/− (green lines), and Vav3−/− (blue lines) cells transduced with either p190-BCR-ABL (solid lines) or mock vector (MIEG3, dotted lines). (B) S-phase fraction as assessed by bromodeoxyuridine incorporation of B-cell progenitors expressing either mock vector (hatched bars) or p190-BCR-ABL (solid bars) on day 6 of in vitro culture. (C) Apoptosis as assessed by annexin V binding of leukemic p190-BCR-ABL+ B-cell progenitors on day 16 of in vitro culture. (D) Representative example of immunoprecipitation and Western blot of pTyr-Vav and total Vav3 in p190-BCR-ABL+ B-cell progenitors from WT, Vav1−/−;Vav2−/−, and Vav3−/− mice. Lysates from 5 × 105 B-cell progenitors were immunoprecipitated, loaded, and blotted with Abs against phospho-tyrosine and Vav3. β-actin expression analysis from total lysate was used as a loading control (n = 3 independent experiments).
To analyze the cellular mechanism responsible for the differential effect of Vav3 and Vav1/Vav2 deficiencies on the expansion of leukemic B-cell progenitors, we determined the proliferation and survival of leukemic-transformed and nonleukemic-transformed B-cell progenitors in vitro. We found that the absence of Vav3 was associated with decreased cell-cycle progression (Figure 2B and supplemental Figure 2). In contrast, combined Vav1 and Vav2 deficiency did not affect the proliferation of p190-BCR-ABL+ B-cell progenitors significantly (Figure 2B). Interestingly, the deficiency of Vav3 or Vav1/Vav2 did not modify cell-cycle entry of mock-transduced B-cell progenitors significantly (Figure 2A-B and supplemental Figure 2), suggesting that p190-BCR-ABL uses Vav3 as a signal mediator in cell proliferation. As expected, p190-BCR-ABL expression enhanced survival of B-cell progenitors in culture over 2 weeks (cell death in mock-transduced B-cell progenitors > 95%, Figure 2A and supplemental Figure 3A). Vav3 deficiency impaired survival of p190-BCR-ABL B-cell progenitors significantly, as assessed in liquid culture (Figure 2C) and in CFU-proB cultures (supplemental Figure 3B-D), whereas combined Vav1/Vav2 deficiency was associated with significantly decreased leukemic progenitor death (Figure 2C and supplemental Figure 3B-D). These data indicate that Vav3 controls the proliferation and survival of leukemic lymphoid progenitors and that the survival of leukemic B-cell progenitors is inversely affected by the absence of Vav1/Vav2 expression.
Because Vav function may be subject to compensatory regulation by the other Vav proteins, as suggested by the up-regulation of Vav1 and Vav2 expression in Vav3-deficient B-cell progenitors (supplemental Figure 1A-C), we analyzed whether the combined deficiency of Vav1 and Vav2 modified Vav3 expression and/or activation significantly. We found that the combined deficiency of Vav1 and Vav2 up-regulated Vav3 expression (supplemental Figure 3E) and tyrosine phosphorylation (Figure 2D), as assessed in whole-cell lysates and Vav3-immunoprecipitated specimens, respectively. These data imply that Vav3 activation is responsible for the changes in cell-cycle progression and survival and suggest that the increased survival observed in Vav1/Vav2–deficient, p190-BCR-ABL–transduced B-cell progenitors is due to compensatory overexpression and overactivation of Vav3. We also analyzed whether Vav3 and BCR-ABL coimmunoprecipitated in leukemic cells. Vav3 and c-Abl immunoprecipitates of Ba/F3 cells transduced with either p190-or p210-BCR-ABL did not show any significant binding of Vav3 and BCR-ABL (supplemental Figure 4A-B), suggesting that BCR-ABL–induced Vav3 activation does not depend on direct binding.
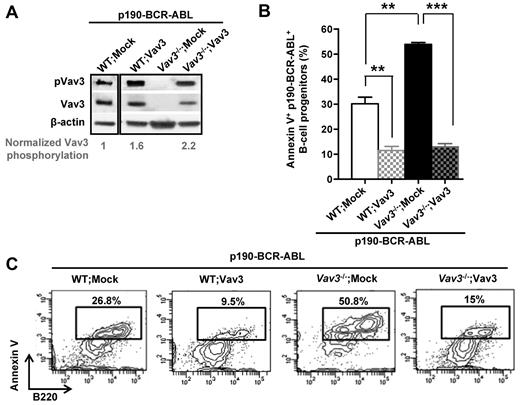
To confirm the functional role of Vav3 in survival of leukemic B-cell progenitors, we introduced Vav3 cDNA into Vav3−/−, p190-BCR-ABL+ B-cell progenitors. Transduction of Vav3−/− p190-BCR-ABL+ B-cell progenitors with a lentiviral vector expressing Vav3 (denoted Vav3−/−;Vav3 in Figure 3A) resulted in restoration of Vav3 activation. Reintroduction of Vav3 reversed the state of apoptosis induced by Vav3 deficiency significantly in p190-BCR-ABL+ B-cell progenitors to a level similar to that in WT leukemic cells (Figure 3B-C).
Expression of transgenic Vav3 rescues Vav3-deficient, p190-BCR-ABL+ leukemogenic cell survival. (A) Representative example (n = 4) of pVav3 and Vav3 expression in WT or Vav3−/− p190-BCR-ABL+ B-cell progenitors transduced with either empty vector (WT; Mock or Vav3−/−; Mock) or Vav3 (WT;Vav3 or Vav3−/−;Vav3). The level of Vav3 phosphorylation (pVav3/Vav3 density ratio) is presented normalized to the WT;Mock value. (B) Apoptosis as determined by annexin V binding of transduced B-cell progenitors (day 15 of culture). **P < .005; ***P < .001. Each experiment was done in triplicate and data are represented as means ± SD. (C) Representative example of FACS analysis of annexin V binding.
Expression of transgenic Vav3 rescues Vav3-deficient, p190-BCR-ABL+ leukemogenic cell survival. (A) Representative example (n = 4) of pVav3 and Vav3 expression in WT or Vav3−/− p190-BCR-ABL+ B-cell progenitors transduced with either empty vector (WT; Mock or Vav3−/−; Mock) or Vav3 (WT;Vav3 or Vav3−/−;Vav3). The level of Vav3 phosphorylation (pVav3/Vav3 density ratio) is presented normalized to the WT;Mock value. (B) Apoptosis as determined by annexin V binding of transduced B-cell progenitors (day 15 of culture). **P < .005; ***P < .001. Each experiment was done in triplicate and data are represented as means ± SD. (C) Representative example of FACS analysis of annexin V binding.
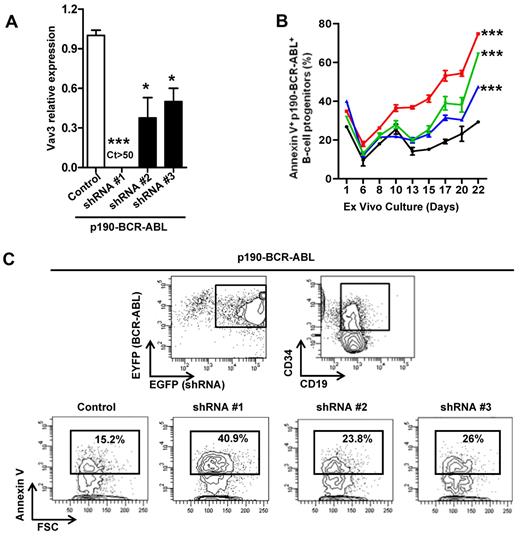
To verify the relevance of our findings in murine leukemic cells, human UCB CD34+ cells were retrovirally transduced with a p190-BCR-ABL–internal ribosomal entry site (IRES)–EYEP vector and 3 different Vav3 shRNA-IRES-EGFP–containing vectors and a nontargeting shRNA-IRES-EGFP–containing vector as a control. The level of interference of Vav3 expression was confirmed in sorted EGFP+/EYFP+/CD34+/CD19+ cells (Figure 4A). Increased levels of apoptosis of ex vivo–cultured cells were inversely correlated with the level of expression of Vav3 (Figure 4B-C). These data support the conclusion that Vav3 is required for p190-BCR-ABL+ murine and human leukemic cell survival and that its activation may not depend on direct protein-protein interaction.
Level of Vav3 expression is correlated with cell death of p190-BCR-ABL transduced human CD34+ cells. (A) Relative Vav3 mRNA expression was assessed by real-time quantitative RT-PCR. Three different shRNA-containing vectors were lentivirally transduced and different levels of Vav3 gene interference were detected cycle threshold (Ct > 50, undetectable level). (B) Apoptosis as determined by annexin V binding in shRNA-transduced p190-BCR-ABL+ human B-cell progenitors (day 15 of culture). ***P < .001 from day 10 onward for shRNA #1 (red symbols and line) and from day 13 onward for shRNA #2 (green symbols and line) and shRNA #3 (blue symbols and line) versus control (ANOVA test). Black symbols and line denote control shRNA values. (C) Representative example of FACS analysis of annexin V binding. Annexin V binding was analyzed on EGFP (shRNA)+/EYFP (p190-BCR-ABL)+/CD19+/CD34+ cells.
Level of Vav3 expression is correlated with cell death of p190-BCR-ABL transduced human CD34+ cells. (A) Relative Vav3 mRNA expression was assessed by real-time quantitative RT-PCR. Three different shRNA-containing vectors were lentivirally transduced and different levels of Vav3 gene interference were detected cycle threshold (Ct > 50, undetectable level). (B) Apoptosis as determined by annexin V binding in shRNA-transduced p190-BCR-ABL+ human B-cell progenitors (day 15 of culture). ***P < .001 from day 10 onward for shRNA #1 (red symbols and line) and from day 13 onward for shRNA #2 (green symbols and line) and shRNA #3 (blue symbols and line) versus control (ANOVA test). Black symbols and line denote control shRNA values. (C) Representative example of FACS analysis of annexin V binding. Annexin V binding was analyzed on EGFP (shRNA)+/EYFP (p190-BCR-ABL)+/CD19+/CD34+ cells.
Vav3 is required for complete leukemogenesis induced by p190-BCR-ABL
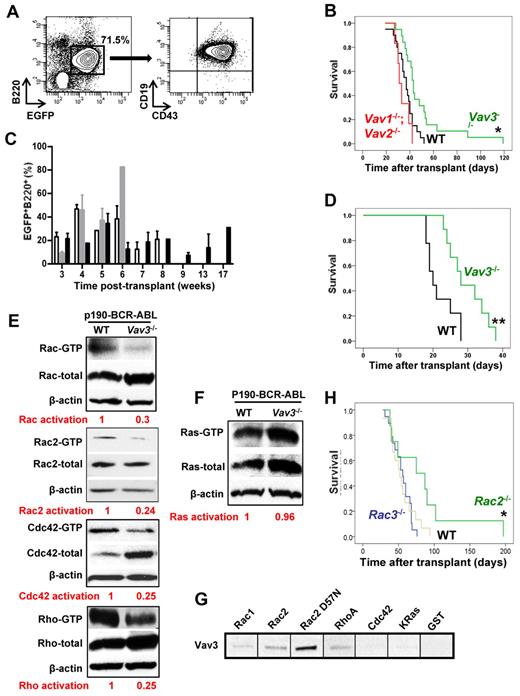
As a consequence of these results, we next determined the effect of Vav3 expression on p190-BCR-ABL+ B-ALL leukemogenesis in vivo. Because loss of Vav proteins has been associated with defective BCR signaling and defective naive B-cell survival,16 we first determined whether there were significant quantitative or functional defects in the BM B-cell progenitor content of Vav-deficient mice that may have impaired the development of B-cell lymphopoiesis at an early stage of differentiation. Vav3−/− and Vav1−/−;Vav2−/− mice had a normal numbers of immunophenotypically defined B-cell progenitors (supplemental Figure 5A). Functional analysis also confirmed normal numbers of progenitors (supplemental Figure 5B). When mice were transplanted with LDBM cells transduced with a retrovirus vector expressing p190-BCR-ABL, greater than 90% of recipient mice developed B-ALL. These mice maintained an expanded B220dim+, CD19+, CD43+/dim B-cell progenitor population in circulation (Figure 5A). B-ALL mice showed both circulation and organ infiltration of p190-BCR-ABL+ lymphoid cells leading to splenomegaly, lymphadenopathy, and CNS manifestations characterized by limb palsies. The mean survival of congenic C57Bl/10 mice transplanted with WT, p190-BCR-ABL+ LDBM was 36.5 ± 1.8 days. Survival was significantly extended to 49.4 ± 4.9 days in mice transplanted with Vav3−/−, p190-BCR-ABL+ LDBM (Figure 5B, log-rank test, P < .05). In contrast, loss of Vav1 (supplemental Figure 6) or combined deletion of Vav1 and Vav2 did not prolong the survival of recipient mice (33.7 ± 2.3 days; Figure 5B, P = not significant.). All recipient mice maintained peripheral B220+/EGFP+ cells from day +21 after transplantation throughout the study, confirming sustained engraftment of p190-BCR-ABL–expressing B-lymphoid cells even in the absence of Vav proteins (Figure 5C). Moreover, impaired leukemogenesis of sorted BM Vav3-deficient B-cell progenitors (EGFP+/ B220+/cKit+) was confirmed in secondary recipients whose survival was significantly extended (29.7 ± 1.8 days) compared with secondary recipients of WT leukemic cells (21.8 ± 1.4 days, P < .01, Figure 5D). Interestingly, mice transplanted with Vav3-deficient, p210-BCR-ABL–transduced BM cells in which Vav3 was also activated (supplemental Figure 7A) developed similar phenotypically defined leukemias as mice transplanted with p190-BCR-ABL (supplemental Figure 7B-C). However, the survival of Vav3−/− p210-BCR-ABL recipients was similar to that of mice transplanted with WT p210-BCR-ABL leukemic cells (supplemental Figure 7D).
Vav3 deficiency delays p190-BCR-ABL induced B-ALL leukemogenesis and is associated with reduced activation of Rac. (A) Circulating p190-BCR-ABL–expressing B-cell progenitors in murine B-ALL transduction/transplantation model as determined by FACS analysis. (B) Survival of mice transplanted with 3 × 106 p190-BCR-ABL+ LDBM cells from WT (n = 22, black), Vav3−/− (n = 19, green), or Vav1−/−;Vav2−/− (n = 6, red) mice. *P < .05 (log-rank test). (C) Percentage of EGFP+/B220+ cells in the peripheral blood of WT (C57Bl/10, empty bars), Vav1−/−;Vav2−/− (gray bars) or Vav3−/− (black bars) mice shown in Figure 1B at different time points. (D) Representative experiment of 2 experiments with similar results, showing survival of secondary mice transplanted with sorted leukemic B-cell progenitors from primary B-ALL mice engrafted with p190-BCR-ABL WT (black) or p190-BCR-ABL, Vav3-deficient (green) cells. EGFP+B220+CD43+, cKitdim+ cells 4 × 105) were isolated and transplanted along 5 × 106 normal congenic BM cells into secondary recipients (C57Bl/10, n = 9 for each group). **P < .01 (log-rank test). (E-F) Representative examples of effector binding domain pull-down assays for total Rac, Rac2, Cdc42, and Rho (E) and Ras (F) of p190-BCR-ABL–expressing murine B-ALL BM cells (n = 3 independent experiments). Relative activation ratio (GTP-bound GTPase/total GTPase) normalized to the WT value is provided below each panel. (G) Representative example of Vav3 (effector) binding assays. Lysate from p190-BCR-ABL+ BaF/3 was incubated with GST alone, GST-fused Rac1, Rac2, Rac2D57N, Cdc42, or K-Ras (2 μg) and 10 μL of suspended glutathione-agarose (n = 3 independent experiments). (H) Kaplan-Meier survival curves of mice transplanted with p190 BCR-ABL–transduced LDBM cells from Rac2−/− (n = 10, green line), Rac3−/− (n = 22, blue line), or WT mice (n = 17, brown line). *P < .05 (log-rank test).
Vav3 deficiency delays p190-BCR-ABL induced B-ALL leukemogenesis and is associated with reduced activation of Rac. (A) Circulating p190-BCR-ABL–expressing B-cell progenitors in murine B-ALL transduction/transplantation model as determined by FACS analysis. (B) Survival of mice transplanted with 3 × 106 p190-BCR-ABL+ LDBM cells from WT (n = 22, black), Vav3−/− (n = 19, green), or Vav1−/−;Vav2−/− (n = 6, red) mice. *P < .05 (log-rank test). (C) Percentage of EGFP+/B220+ cells in the peripheral blood of WT (C57Bl/10, empty bars), Vav1−/−;Vav2−/− (gray bars) or Vav3−/− (black bars) mice shown in Figure 1B at different time points. (D) Representative experiment of 2 experiments with similar results, showing survival of secondary mice transplanted with sorted leukemic B-cell progenitors from primary B-ALL mice engrafted with p190-BCR-ABL WT (black) or p190-BCR-ABL, Vav3-deficient (green) cells. EGFP+B220+CD43+, cKitdim+ cells 4 × 105) were isolated and transplanted along 5 × 106 normal congenic BM cells into secondary recipients (C57Bl/10, n = 9 for each group). **P < .01 (log-rank test). (E-F) Representative examples of effector binding domain pull-down assays for total Rac, Rac2, Cdc42, and Rho (E) and Ras (F) of p190-BCR-ABL–expressing murine B-ALL BM cells (n = 3 independent experiments). Relative activation ratio (GTP-bound GTPase/total GTPase) normalized to the WT value is provided below each panel. (G) Representative example of Vav3 (effector) binding assays. Lysate from p190-BCR-ABL+ BaF/3 was incubated with GST alone, GST-fused Rac1, Rac2, Rac2D57N, Cdc42, or K-Ras (2 μg) and 10 μL of suspended glutathione-agarose (n = 3 independent experiments). (H) Kaplan-Meier survival curves of mice transplanted with p190 BCR-ABL–transduced LDBM cells from Rac2−/− (n = 10, green line), Rac3−/− (n = 22, blue line), or WT mice (n = 17, brown line). *P < .05 (log-rank test).
To confirm the dispensable role of Vav3 in human B-ALL induced by p210-BCR-ABL, we retrovirally transduced human UCB CD34+ cells with p210-BCR-ABL and with Vav3 shRNA-containing lentiviral vectors. Similar to p190-BCR-ABL–transduced CD34+/ CD19+ cells, Vav3 expression in shRNA–transduced, p210-BCR-ABL–expressing CD34+/CD19+ cells was also silenced (supplemental Figure 8A). However, the level of p210-BCR-ABL+ leukemic cell apoptosis was not changed (supplemental Figure 8B-C), suggesting that the Dbl- and/or pleckstrin-homology domains of p210-BCR-ABL may overcome the deficiency of Vav3 activation and that Vav3 activation may be dispensable for p210-BCR-ABL leukemogenesis. These data suggest that Vav3, but not Vav1 or Vav2, is specifically required for leukemogenesis induced by p190-BCR-ABL.
Vav3 deficiency impairs the Rac GTPase signaling pathway in vivo
Both p190 and p210 forms of BCR-ABL activate small GTPases, including Rac, Rho, and Ras GTPases (supplemental Figure 9). We then analyzed whether Vav3 is required for activation of small GTPases in p190-BCR-ABL expressing B-cell progenitors. We observed that the activation of total-Rac, Rac-2, Cdc42 and Rho were diminished in the absence of Vav3 in B-ALL cells harvested from murine leukemias (Figure 5E). On the other hand, the activation of Ras was not reduced in Vav3-deficient p190-BCR-ABL+ leukemic B-cell progenitors (Figure 5F). This result is consistent with previous observations that Ras is not a direct substrate of Vav.22 These data also indicate that Vav3 is necessary for full activation of Rho GTPases by p190-BCR-ABL in B-lymphoid progenitors.
Among the Rho GTPases, we showed previously that Rac, especially Rac2, is critical in the proliferation and survival of p210-BCR-ABL+ CML leukemic stem cells,9,10 whereas the single deficiency of Rac1 did not impair their leukemogenic ability in vivo.9 To determine whether Vav3 plays a role as a GEF for Rac2 in p190-BCR-ABL B-cell progenitors, we performed an effector pull-down assay of GST-bound recombinant small GTPases. We found that Rac2 to a higher degree, Rac1, Rho, and Cdc42 only marginally bind Vav3 from lymphoblastic leukemic p190-BCR-ABL–expressing cells (Figure 5G). K-Ras did not bind Vav3 (Figure 5G). A dominant-negative mutant of Rac2 (Rac2D57N, nucleotide-free and with an ability to act as a GEF sink)23 has increased binding to Vav3 (Figure 5G). In combination with the small GTPase pull-down data (Figure 5E-F), these data strongly suggest that Rac/Rho, and specifically Rac2, bind and are activated by Vav3 in p190-BCR-ABL B-cell progenitors.
To determine the role of p190-BCR-ABL–induced Rac activation in leukemogenesis, we analyzed the role of Rac2, which strongly binds to Vav3 (Figure 5G), and Rac3, which has been implicated in BCR-ABL leukemia,24 in in vivo leukemogenesis. Deficiency of Rac2, but not Rac3, delayed p190-BCR-ABL leukemogenesis and prolonged mean survival time (54.6 ± 3.0, 84.8 ± 18.2, and 54.9 ± 4.6 days for mice transplanted with WT, Rac2−/−, and Rac3−/− p190-BCR-ABL–transduced LDBM, respectively, P < .05 for comparison between mice engrafted with WT vs Rac2−/− LDBM; Figure 5H). Similar to previous data in a model of p210-BCR-ABL myeloproliferative disease,9 Rac2-deficient mice showed levels of EGFP+/B220+ cells in peripheral blood comparable with that in recipients of WT-transduced cells until their death (supplemental Figure 10). The contents of B-cell progenitors in BM of Rac2−/− donor mice was normal, suggesting that the lack of a target cell population was not responsible for the delayed latency of leukemia (supplemental Figure 11A-B). These data confirm the role of Rac2 in p190-BCR-ABL–induced leukemogenesis and phenocopy the survival advantage of Vav3-deficient leukemias.
Vav3 is required to maintain the balance between survival and death signals in p190-BCR-ABL+ B-ALL
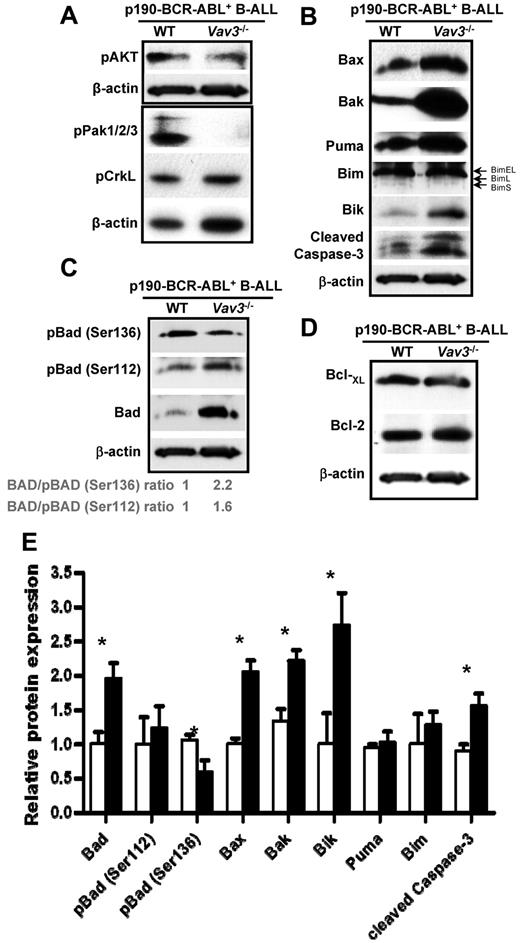
To address the signaling pathways responsible for Vav3-dependent leukemic B-cell progenitor survival, we explored the molecular mechanism of increased apoptosis in Vav3−/− leukemic B-cell progenitors. Whereas activation of Akt was not consistently altered by Vav3 deficiency (Figure 6A), activation of p21-activated kinase (PAK), a direct effector of Rac GTPases, was decreased markedly in p190-BCR-ABL+ Vav3−/− B-cell progenitors (Figure 6A). In contrast, the activation of the adaptor protein CrkL, which binds ABL directly, was not changed in the absence of Vav3 (Figure 6A). BCR-ABL–dependent signals are known to modulate the prosurvival proteins of the Bcl-2 family. Bcl2 family members have been suggested as therapeutic targets in BCR-ABL leukemias.25 To determine whether Vav3-dependent survival of p190-BCR-ABL+ B-ALL cells was mediated by Bcl2 family proteins, we analyzed the effect of Vav3 deficiency on the expression of Bcl2 family in primary p190 BCR-ABL+ leukemic B-cell progenitors from B-ALL mice. There was a significant increase in the expression of death signals in Vav3-deficient, p190-BCR-ABL+ B-cell progenitors in vitro (supplemental Figure 12) and leukemic blasts in vivo (Figure 6B-C,E). Vav3−/− p190-BCR-ABL+ B-ALL cells showed increased expression of cleaved caspase-3, confirming apoptotic cell death associated with increased expression of the proapoptotic proteins Bax, Bak, and the BH3-only molecule Bik (Figure 6B,E). Although the level of expression of Bim, another BH3-only molecule, was not changed in Vav3-deficient B-ALL primary tumors (Figure 6B), the expression of all 3 isoforms of Bim was up-regulated significantly in cultured p190-BCR-ABL+ B-cell progenitors (supplemental Figure 12), suggesting that an interplay of in vivo signals may reverse Bim up-regulation induced by Vav3 loss of function. In addition, Bad mRNA and protein expression was increased markedly in Vav3-deficient, p190-BCR-ABL+ B-cell progenitors (Figure 6C,E and supplemental Figure 13). Bad inactivation prevents association of Bad with Bcl2/Bcl-xL and promotes cell survival. To address whether Vav3 deficiency is associated with inactivation of Bad, we analyzed 2 specific sites of Bad phosphorylation that result from alternative kinase pathways. The level of phospho(Ser136)-Bad which is the result of PAK/Akt pathway activation,26,27 was reduced significantly (Figure 6C,E), in agreement with decreased PAK activation. However, the level of phospho(Ser112)-Bad, which is dependent on p90RSK28 and protein kinase A activation,29 was not changed (Figure 6C,E). The expression of antiapoptotic Bcl-xL and Bcl-2 were similar in Vav3−/−p190-BCR-ABL+ B-ALL cells compared with their WT counterparts (Figure 6D). These data indicate Vav3 modulates the balance between pro- and antiapoptotic signals in p190-BCR-ABL–expressing leukemia cells. Moreover, these results suggest that the up-regulation of BH3-only molecules induced by the loss of Vav3 can counteract the increased survival of leukemic cells resulting from BCR-ABL–dependent activation of prosurvival Bcl-2 family members.
Vav3 modulates Bcl-2 family for p190-BCR-ABL+ leukemic cell survival. (A) Representative immunoblots of p-AKT, p-PAK, and p-CrkL in B-cell progenitors from primary murine B-ALL BM cells. β-actin expression analysis was used as a loading control. (B) Representative immunoblots of Bax, Bak, Puma, Bim (EL, L, and S isoforms are identified), and Bik in p190-BCR-ABL+ B-cell progenitors. Cleaved caspase-3 expression was analyzed to confirm proapoptotic status of analyzed cells and β-actin was used as a loading control. (C) Level of expression and phosphorylation of Bad in WT and Vav3−/− leukemic B-cell progenitors. (D) Representative immunoblots of Bcl-xL and Bcl-2 in p190-BCR-ABL+ WT or Vav3−/− B-ALL murine BM cells. β-actin was used as a loading control (n = 3 independent experiments). (E) Normalized expression levels of proteins presented in panels B and C (n = 3 independent experiments). Vav3-deficient, p190-BCR-ABL+ primary B-ALL BM cells (solid bars) were normalized to their WT counterparts (empty bars). Results are shown as means ± SD. *P < .05.
Vav3 modulates Bcl-2 family for p190-BCR-ABL+ leukemic cell survival. (A) Representative immunoblots of p-AKT, p-PAK, and p-CrkL in B-cell progenitors from primary murine B-ALL BM cells. β-actin expression analysis was used as a loading control. (B) Representative immunoblots of Bax, Bak, Puma, Bim (EL, L, and S isoforms are identified), and Bik in p190-BCR-ABL+ B-cell progenitors. Cleaved caspase-3 expression was analyzed to confirm proapoptotic status of analyzed cells and β-actin was used as a loading control. (C) Level of expression and phosphorylation of Bad in WT and Vav3−/− leukemic B-cell progenitors. (D) Representative immunoblots of Bcl-xL and Bcl-2 in p190-BCR-ABL+ WT or Vav3−/− B-ALL murine BM cells. β-actin was used as a loading control (n = 3 independent experiments). (E) Normalized expression levels of proteins presented in panels B and C (n = 3 independent experiments). Vav3-deficient, p190-BCR-ABL+ primary B-ALL BM cells (solid bars) were normalized to their WT counterparts (empty bars). Results are shown as means ± SD. *P < .05.
Vav3 deficiency collaborates with TKIs to impair survival of leukemic lymphoid progenitors in vitro and in vivo
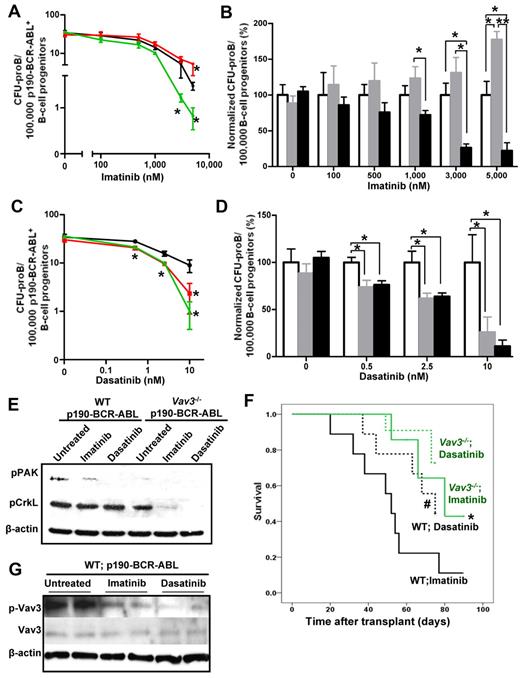
BCR-ABL TK activity is considered to be responsible for BCR-ABL transformation. However, TKIs targeting BCR-ABL have only shown a transient and modest effect on the long-term survival of BCR-ABL+ B-ALL patients.30 Therefore, we examined whether TK inhibition and deficiency of Vav proteins collaborated in terms of inhibition of p190-BCR-ABL+ leukemic cell proliferation while cultured in presence of SCF and IL-7. To allow similar numbers of starting CFU-proB cells in the original inocula, p190-BCR-ABL–transduced LDBM cells were cultured for 3 days (a selected period of time when Vav3 deficiency has not yet induced a loss of B-cell progenitors, as demonstrated in Figure 2A), then sorted for EGFP+ B-cell progenitors and plated in methylcellulose with different concentrations of imatinib or dasatinib. Loss of Vav3 collaborated with TK inhibition by imatinib (Figure 7A-B) at doses of 1μM or higher to inhibit B-cell progenitor growth. In contrast, the combined deficiency of Vav1 and Vav2 attenuated the effect of imatinib on B-cell lymphoid progenitor growth and demonstrated an antagonistic effect (Figure 7A-B), which is in agreement with our previous data on the pathogenic role of activated Vav3 in the context of Vav1/Vav2 combined deficiency. Vav3 deficiency also collaborated with dasatinib, which is a combined BCR-ABL and Src family inhibitor, to prevent the growth of leukemic B-lymphoid progenitors (Figure 7C-D). Interestingly, the combined deficiency of Vav1 and Vav2 also collaborated with dasatinib to inhibit B-cell progenitor outgrowth (Figure 7C-D), suggesting that the Src family kinase inhibitory activity of dasatinib may antagonize the function of hyperactivated Vav3.
Loss of Vav3 sensitizes p190 BCR-ABL+ B-cell progenitors to kinase inhibitor drug treatments in vivo and in vitro. (A) B-cell progenitor frequency (CFU-proB) of p190-BCR-ABL–transduced LDBM cells in the presence or absence of different concentrations of imatinib. Transduced LDBM cells were cultured for 9 days in methylcellulose with the indicated doses of imatinib. Results are shown as means ± SD of 2 independent experiments performed in triplicate. *P < .05. (B) Normalized B-cell progenitor cell frequency with respect to WT cells from the same cultures shown in panel A. *P < .05; **P < .01. (C) B-cell progenitor frequency (CFU-proB) pf p190-BCR-ABL–transduced LDBM cells in the presence or absence of different concentrations of dasatinib carried out as in panel A. Two independent experiments were performed in triplicate. *P < .05. (D) Normalized B-cell progenitor frequency (CFU-proB) with respect to WT cells from the same cultures shown in panel C. B-cell progenitor frequencies of WT cells are depicted by black lines (A,C) or empty bars (B,D); Vav1−/−;Vav2−/− are depicted by red lines (A,C) or gray bars (B,D); and Vav3−/− are depicted by green lines (A,C) or black bars (B,D). (E) Representative example of immunoblot of p-PAK and p-CrkL in sorted B-cell progenitors from primary mouse LDBM cells transduced with p190-BCR-ABL. Sorted leukemic B-cell progenitors were pretreated with 5μM imatinib, 10nM dasatinib, or no drug (untreated) for 2 hours in vitro. β-actin expression was analyzed as a loading control (n = 2 independent experiments). (F) Kaplan-Meier survival curves of p190-BCR-ABL–induced B-ALL mice treated with either imatinib (solid lines) or dasatinib (dotted lines). WT (black solid line, n = 13) and Vav3-deficient (green solid line, n = 13), p190-BCR-ABL+ B-ALL mice were treated with daily doses of imatinib for up to 90 days. WT (black dotted line, n = 10) and Vav3-deficient (green dotted line, n = 15), p190-BCR-ABL+ B-ALL mice were treated with dasatinib for 75 days. *P < .05 between imatinib-treated WT B-ALL mice and Vav3−/− B-ALL mice; #P < .05 between imatinib-treated WT B-ALL mice and dasatinib-treated WT B-ALL mice (log-rank test). (G) Representative examples of immunoblot of Vav3 activation (p-Vav3) and expression (Vav3) in EGFP+ B cells from the BM of leukemic animals transplanted with WT, p190-BCR-ABL–expressing BM cells and left untreated or treated with imatinib or dasatinib (n = 2 mice per group in 1 of 2 independent experiments).
Loss of Vav3 sensitizes p190 BCR-ABL+ B-cell progenitors to kinase inhibitor drug treatments in vivo and in vitro. (A) B-cell progenitor frequency (CFU-proB) of p190-BCR-ABL–transduced LDBM cells in the presence or absence of different concentrations of imatinib. Transduced LDBM cells were cultured for 9 days in methylcellulose with the indicated doses of imatinib. Results are shown as means ± SD of 2 independent experiments performed in triplicate. *P < .05. (B) Normalized B-cell progenitor cell frequency with respect to WT cells from the same cultures shown in panel A. *P < .05; **P < .01. (C) B-cell progenitor frequency (CFU-proB) pf p190-BCR-ABL–transduced LDBM cells in the presence or absence of different concentrations of dasatinib carried out as in panel A. Two independent experiments were performed in triplicate. *P < .05. (D) Normalized B-cell progenitor frequency (CFU-proB) with respect to WT cells from the same cultures shown in panel C. B-cell progenitor frequencies of WT cells are depicted by black lines (A,C) or empty bars (B,D); Vav1−/−;Vav2−/− are depicted by red lines (A,C) or gray bars (B,D); and Vav3−/− are depicted by green lines (A,C) or black bars (B,D). (E) Representative example of immunoblot of p-PAK and p-CrkL in sorted B-cell progenitors from primary mouse LDBM cells transduced with p190-BCR-ABL. Sorted leukemic B-cell progenitors were pretreated with 5μM imatinib, 10nM dasatinib, or no drug (untreated) for 2 hours in vitro. β-actin expression was analyzed as a loading control (n = 2 independent experiments). (F) Kaplan-Meier survival curves of p190-BCR-ABL–induced B-ALL mice treated with either imatinib (solid lines) or dasatinib (dotted lines). WT (black solid line, n = 13) and Vav3-deficient (green solid line, n = 13), p190-BCR-ABL+ B-ALL mice were treated with daily doses of imatinib for up to 90 days. WT (black dotted line, n = 10) and Vav3-deficient (green dotted line, n = 15), p190-BCR-ABL+ B-ALL mice were treated with dasatinib for 75 days. *P < .05 between imatinib-treated WT B-ALL mice and Vav3−/− B-ALL mice; #P < .05 between imatinib-treated WT B-ALL mice and dasatinib-treated WT B-ALL mice (log-rank test). (G) Representative examples of immunoblot of Vav3 activation (p-Vav3) and expression (Vav3) in EGFP+ B cells from the BM of leukemic animals transplanted with WT, p190-BCR-ABL–expressing BM cells and left untreated or treated with imatinib or dasatinib (n = 2 mice per group in 1 of 2 independent experiments).
To understand mechanistically the signaling mechanisms of collaboration between Vav3 and the TK domain of ABL, we analyzed the activation of Pak and CrkL, which has been shown to be crucial in BCR-ABL–induced leukemogenesis,31 in TKI-treated, sorted leukemic B-cell progenitors. Both Vav3 deficiency and incubation with TKIs are highly effective in impairing Pak activation in lymphoblastic progenitors, and seem to have an additive effect (Figure 7E). Unlike TKIs32 or Vav3 deficiency alone, incubation with either imatinib or dasatinib synergized with Vav3 deficiency to completely abrogate activation of CrkL (Figure 7E). These data strongly suggest that the TK activity of p190-BCR-ABL and Vav3 activity collaborate in Pak activation and that they are functionally redundant for activation of CrkL.
Collaboration of Vav3 deficiency and administration of TKIs was also analyzed in vivo. Vav3 deficiency collaborated with administration of imatinib to extend the survival of mice transplanted with either WT or Vav3−/− p190-BCR-ABL–transduced LDBM cells (mean survivals times, 52.0 ± 6.8 days and 77.3 ± 5.7 days, respectively, P < .05; Figure 7F) compared with mice receiving no TKI administration (Figure 5B). Whereas dasatinib administration extended the survival of mice transplanted with WT, p190-BCR-ABL–transduced LDBM cells significantly (average survival time, 65.2 ± 5.2 days) compared with mice treated with imatinib (P < .05), the effect of Vav3 deficiency (average survival time, 72.3 ± 2.2 days) was more modest and did not reach statistical significance (Figure 7F). Analysis of Vav3 activation in leukemic EGFP+ BM cells from imatinib- and dasatinib-treated WT deceased animals demonstrated a modest inhibition of Vav3 activation, which was more significant for dasatinib-treated leukemias, albeit not abrogated (Figure 7G). Vav3 expression was not modified by imatinib or dasatinib treatment. These data suggest that Vav3 activation in vivo may depend on multiple signals, including the ABL TK of p190-BCR-ABL and Src kinase activity.
The results of the present study suggest that TKI therapy does not suffice to prevent Vav3 activation and that Vav3 deficiency collaborates with TKIs to inhibit Pak and CrkL to inhibit leukemogenesis in vitro and in vivo.
Discussion
Our previous studies have demonstrated that hyperactivation of Rac GTPases, and specifically Rac2, are key signals in BCR-ABL–induced transformation and leukemogenesis.9,10 Lymphoid transformation of BCR-ABL–expressing progenitors has been associated with genomic instability and mutagenesis through mechanisms that are not well characterized but in which Rac activation has been suggested to play a role through the generation of reactive oxygen species.12
In the presents tudy, we explored the upstream mechanism of Rac activation by the short form of BCR-ABL, which lacks an activating DH domain. We found that Vav3 plays a crucial role in p190-BCR-ABL–mediated leukemogenesis, proliferation, and survival. Our data show that p190-BCR-ABL up-regulates both Vav1 and Vav3 expression and activation, but only the deficiency of Vav3 attenuates the transformation phenotype regarding progenitor proliferation, especially the survival of B-cell progenitors, the putative leukemia-initiating cells in this disease. Vav3 deficiency delays p190-BCR-ABL–induced but not p210-BCR-ABL–induced lymphoid leukemogenesis in vivo. The delay in lymphoid leukemogenesis induced by the loss of Vav3 phenocopies the deficiency of Rac2, indicating that whereas the functional relevance of Vav3 activation relates specifically to p190-BCR-ABL but not p210-BCR-ABL expression, the dependence on Rac2 activity seems to affect to both p210-BCR-ABL leukemia9,10 and p190-BCR-ABL leukemia (Figure 5H). Vav3 deficiency impairs cell-cycle progression and survival mediated by p190-BCR-ABL, unlike a combined deficiency of Vav1 and Vav2, which leads to increased Vav3 activation with either no effect or modest enhancement of leukemic transforming phenotypes. Moreover, the survival impairment induced by Vav3 deficiency can be rescued by exogenous expression of Vav3 in Vav3−/− lymphoid progenitors, ruling out a developmental defect associated with Vav3 deficiency in primary murine cells.
To understand the role of Vav3 signaling in p190-BCR-ABL+ cell survival, we investigated whether Vav3 coimmunoprecipitated with BCR-ABL and the downstream pathways of Vav3, specifically the direct effector of Rac GTPase, and Ras activation. First, unlike Vav1,20 we found that Vav3 did not coimmunoprecipitate with either p190-BCR-ABL or p210-BCR-ABL, but as is the case with p210-BCR-ABL leukemia,9,11 Rac GTPases are activated in p190-BCR-ABL leukemia (supplemental Figure 9). Therefore, Vav3 activation seems not to depend on direct binding to BCR-ABL. Second, we found that whereas Ras activity was not affected by Vav3 deficiency, Rac activation was decreased significantly in Vav3-deficient, p190-BCR-ABL lymphoblastic leukemias. Biochemically, Vav3 strongly binds Rac2, Rac1, RhoA, and, marginally, Cdc42 in p190-BCR-ABL–expressing lymphoid leukemic cells. A dominant-negative mutant of Rac2 (Rac2D57N) with significantly impaired GTP binding ability resulting from a markedly enhanced rate of GTP dissociation is able to pull down all Vav3, suggesting that Rac2 is susceptible of being activated by Vav3. Furthermore, Rac2 but not Rac3 deficiency phenocopies Vav3 deficiency in extending the survival of mice transplanted with p190-BCR-ABL–transduced LDBM cells. In agreement with our previously published data on the role of Rac proteins in p210-BCR-ABL+ myeloproliferative disease development9 and the role of Rac2 specifically in leukemic stem cell–initiated myeloproliferative disease,10 the results of the present study further strengthen our understanding of the crucial role of Rac proteins in the initiation and/or progression of BCR-ABL+ leukemias. Although we cannot rule out a non-GEF activity of Vav3 similar to that for Vav1 in other cell contexts,33 our data strongly suggest that Vav3 acts as a GEF of Rac2.
BCR-ABL induces cell proliferation and suppresses apoptosis through different mechanisms, including inhibition of proapoptotic molecules such as Bad.34 Bad controls the life-death switch and promotes apoptosis and cell-cycle arrest by antagonizing the prosurvival and cell-cycle progression activity of Bcl-2/Bcl-xL proteins35 or by activating proapoptotic Bax or Bak directly.36 Bad has been identified as an overexpressed gene in imatinib-resistant CML37 and in Rac2-null mast cells.38 Whereas the expression levels of Bad are important in the regulation of its function, Bad activity also depends on its phosphorylation state. Bad phosphorylation at Ser112 or Ser135/136 induces ubiquitination and protein degradation.39 Our data suggest that Vav3 deficiency regulates cell-cycle arrest and apoptosis that is altered in p190-BCR-ABL leukemogenesis. PAK proteins are major effectors of activated Rac and activated PAK has been shown to phosphorylate and inactivate Bad.40 PAK may also phosphorylate Bik, resulting in the survival of B-lineage lymphoid cells.41 This signal pathway has also shown to be responsible for cytoskeletal rearrangements and cell motility in B-lineage lymphoid cells.42 In the present study, diminished Rac (including Rac2) activation in Vav3-deficient B-ALL was correlated with decreased PAK activation, an increased Bad/pBad ratio, and a deficiency of Rac2 that phenocopied the increased survival of recipient mice of Vav3-deficient, p190-BCR-ABL leukemic cells. In contrast, we found that the expression of Bcl-2 and Bcl-xL was not altered in Vav3-deficient leukemic cells. These data suggest that Vav3 activation may use Rac2/PAK/pBAD as a signaling pathway in p190-BCR-ABL leukemic cell survival.
Although the level of inactive phospho(Ser112)-Bad, a consequence of phosphorylation by p90RSK28 or protein kinase A,29 was maintained (Figure 6C,E), the level of phospho(Ser136)-Bad, a target of PAK and Akt kinase activities, was reduced to promote cell death.26 In the context of decreased PAK activation with unchanged Akt activation, these results strongly suggest that PAK/Bad are downstream signaling targets of Vav3 activity in the context of p190-BCR-ABL leukemogenesis. Increased expression of Bad, Bik, Bax, and Bak in Vav3-deficient cells represents additional negative regulation on lymphoid progenitor proliferation and survival. These data suggest that Vav3 deficiency regulates cell-cycle progression and survival negatively, which counteracts the survival signals induced by BCR-ABL.
Current therapeutic strategies in p190-BCR-ABL+ B-ALL are based on combination therapy regimens incorporating continuous imatinib treatment with intensive multiagent chemotherapy, radiotherapy, and stem-cell transplantation in selected patients.43 A poor response and/or resistance to TKI therapy is believed to be due to mutations in ABL. For this reason, current efforts are focused on the development of new drugs with ABL TKI activity against BCR-ABL mutants.44 Dasatinib, a multitargeted kinase inhibitor with specificity for BCR-ABL, Src family kinases, and other TKs, has shown an advantage over classic ABL TKIs through binding to mutation-induced conformational dynamics of the ABL kinase domain.45 Dasatinib shows significant antileukemic activity and is increasingly used in the treatment of BCR-ABL+ B-ALL.46 Unfortunately, like imatinib, long-term durable responses are not observed with single-agent therapy47 and effects on survival largely depend on combinatorial therapies. With both imatinib and dasatinib, resistance to TKI therapy has been shown to occur in a significant number of patients. Among the downstream mechanisms found to be activated in TKI therapy resistance, Bcl-2 overexpression or loss of Bim and Bad expression48 and gene deletion of CDKN2A and CDKN2B in BCR-ABL+ leukemia cell–initiating cell subpopulations has been associated with aggressive leukemic growth.6 These genetic alterations evolve even in presence of effective TKI and are dependent on cytokine signaling,49 suggesting that signals independent of BCR-ABL TK activity may be responsible for cell transformation, proliferation and survival in BCR-ABL+ leukemia.
The results of the present study show that the combination of Vav3 deficiency and imatinib extends animal survival in vivo and results in the inhibition of B-cell progenitor expansion in vitro, even in the presence of lymphoid cytokines. As expected from our previous data, which showed that combined Vav1/Vav2 deficiency induces overexpression and hyperactivation of Vav3, the combined deficiency of Vav1 and Vav2 showed an opposite effect, inducing B-cell progenitor expansion in vitro. Furthermore, the additive effect of imatinib or dasatinib and Vav3 deficiency strongly suggest that Vav3 controls the activation of BCR-ABL–signaling pathways, and loss of Vav3 collaborates with TK inhibition to revert leukemic progenitor transformation. The combined deficiency of Vav1 and Vav2 antagonizes the inhibitory effect of imatinib on B-cell progenitor proliferation, which may be explained by basal Vav3 up-regulation of Vav1/Vav2–deficient leukemic progenitors. Interestingly, dasatinib, an inhibitor of a much broader spectrum of kinase targets, collaborates with the combined deficiency of Vav1 and Vav2, suggesting that the inhibitory activity of dasatinib on targets other than the ABL TK domain may affect active pathways distinctly secondary to increased Vav3 activation. We have found that in vivo administration of imatinib or further dasatinib inhibits leukemic Vav3 activation, but does not abrogate it, indicating that Vav3 activation depends partly on ABL TK and Src kinase activities, but also on other in vivo signals. The unidentified signals may originate in the BM microenvironment because, as we have shown previously,50 the abrogation of the expression of the full BCR-ABL fusion protein induces B-ALL apoptosis and cures leukemia. The mechanism of collaboration between Vav3 and ABL TK signaling in p190-BCR-ABL B-cell progenitors may relate to their concerted action on Pak and CrkL activation. Whereas Vav3 deficiency impairs Pak activation significantly, the addition of TKI completely abrogates its activation. Similarly to incubation with TKIs,32 the isolated deficiency of Vav3 does not inhibit CrkL activation significantly. However, the combination of either imatinib or dasatinib with Vav3 deficiency abrogates CrkL activation completely. These data may provide an explanation for the clinical resistance to TKI observed in p190-BCR-ABL B-ALL.
In summary, the results of the present study show that Vav3 is a pivotal downstream target in p190-BCR-ABL signaling and acts as a critical determinant of leukemic cell survival. Deregulated expression and activation of Vav3 is associated with leukemic transformation. Loss of Vav3 alone resulted in increased leukemic cell death and impaired disease progression in vivo. Targeted specific inhibition of Vav3 activity may lead to the loss of leukemic cells through decreased survival and, together with TKI therapy, may improve the treatment of p190-BCR-ABL+ ALL.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the patients with ALL who provided consent for analysis of their BM specimens, the translational trial and development support laboratory of Cincinnati Children's Hospital Medical Center for providing healthy human BM specimens, the mouse and research flow cytometry core facilities of Cincinnati Children's Hospital Medical Center, Margaret O'Leary for editorial assistance, and Drs Mohammad Azam and Nicolas Nassar at Cincinnati Children's Hospital Medical Center for helpful comments and discussions.
This project was funded in part by the National Institutes of Health (R01 HL087159 and supplement to J.A.C., R01 DK62757 and CA113969 to D.A.W., and R01 CA73735 to X.R.B.); Alex's Lemonade Stand Foundation, Cancer Free Kids Foundation, and the Department of Defense (CM064050 to J.A.C.); the Spanish Ministry of Science and Innovation (SAF2009-07172 and RD06/0020/0001), the Castilla y León Autonomous Government (GR97), and the 7th Framework European Union Program (FP7-HEALTH-2007-A-201862 to X.R.B.).
National Institutes of Health
Authorship
Contribution: K.H.C., A.S.-A., S.S., A.S., and J.A.C. designed and performed the research and analyzed the data; K.H.C. and J.A.C. wrote the manuscript; M.N.M., A.M.F., S.K.D., A.M.K., J.L.A., and R.A.S. performed the research; X.A., J.P.P., M.W.D., Y.Z., and X.R.B. provided the reagents and made critical contributions to the manuscript; and D.A.W. supported the initial research and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Jose A. Cancelas, EHCB Division, CCHMC, 3333 Burnet Ave, Cincinnati, OH 45229; e-mail: jose.cancelas@cchmc.org.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal