These studies address a vexing intellectual problem regarding the use of factor VIIa as a bypassing agent in hemophilia. The activity of factor VIIa, like that of coagulation factors IXa and Xa, is significantly enhanced when bound to its coagulation cofactor. Thus, factor IXa and factor VIIIa form a functional complex in vivo and the absence of either protein results in hemophilia. Similarly, lipid surface factor Xa activity is enhanced several thousand-fold when bound to factor Va and the complex of factor Xa with factor Va is required for physiologic thrombin generation. Because factor VIIa activity is significantly enhanced in the presence of its cofactor, TF, one might suspect that relatively low doses of factor VIIa would saturate available TF and provide the needed hemostatic activity.
In treating hemophilia patients with inhibitors, some of the initial dosing strategies included administering as little as 35 μg/kg factor VIIa. This dose was predicted to lead to levels on the order of 0.5 mg/mL in plasma; a concentration roughly equal to the plasma concentration of factor VII (10nM). A dose of 35 μg/kg was effective at stopping bleeding in some patients but was suboptimal in many others.2 Clinical studies comparing doses of 35 μg/kg against 70 μg/kg, 90 μg/kg, and 120 /kg suggested that increasing dose were, in general, associated with increasing efficacy.2,3 Given that TF is almost certainly limiting in vivo, it was unclear why higher doses gave greater efficacy.
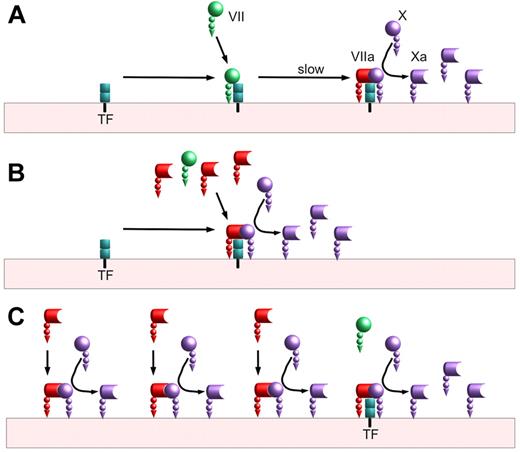
Biochemical studies approached this question and 2 theories emerged (see simplified illustrations in figure). These theories were generally described as TF-dependent4 and TF-independent.5,6 In a sense this is an unfortunate nomenclature; even if factor VIIa at pharmacologic doses has measurable activity independent of TF, the overall coagulation process still requires initiation by TF just as it still requires a surface, calcium, factor X, factor V, and prothrombin. On average, investigators agree that it is biochemically possible for the reactions in both panels B and C of the figure to occur; the 2 theories differ in the degree to which the reactions shown in panel C are relevant to the physiology of high-dose factor VIIa as a therapy.
Factor VIIa activation of factor X. (A) Baseline mechanism: On a lipid surface, factor VII binds to tissue factor (TF) and is slowly activated, either by the 1% of plasma factor VII that is activated or by factor Xa generated by other VIIa/TF complexes. This VIIa/TF complex activates factor X to factor Xa before ultimately being shut off by tissue factor pathway inhibitor (TFPI; not shown). (B) Tissue factor–dependent (competition) mechanism: On a lipid surface, factor VIIa can compete with factor VII for binding to TF. The VIIa/TF complexes can immediately begin activating factor X without requiring a slow activation step. The VIIa/TF complexes can also activate any TF-bound factor VII, further speeding factor X activation. (C) Tissue factor–independent mechanism: In addition to binding to tissue factor, factor VIIa can bind lipid surfaces and convert factor X to factor Xa. This reaction is much slower than the reaction of TF-bound factor VIIa; however, the amount of surface-bound factor VIIa may exceed the amount of TF-bound by orders of magnitude. This reaction is also limited by the amount of available surface. Unlike factor VIIa in a TF complex, factor VIIa without TF is not inhibited by TFPI.
Factor VIIa activation of factor X. (A) Baseline mechanism: On a lipid surface, factor VII binds to tissue factor (TF) and is slowly activated, either by the 1% of plasma factor VII that is activated or by factor Xa generated by other VIIa/TF complexes. This VIIa/TF complex activates factor X to factor Xa before ultimately being shut off by tissue factor pathway inhibitor (TFPI; not shown). (B) Tissue factor–dependent (competition) mechanism: On a lipid surface, factor VIIa can compete with factor VII for binding to TF. The VIIa/TF complexes can immediately begin activating factor X without requiring a slow activation step. The VIIa/TF complexes can also activate any TF-bound factor VII, further speeding factor X activation. (C) Tissue factor–independent mechanism: In addition to binding to tissue factor, factor VIIa can bind lipid surfaces and convert factor X to factor Xa. This reaction is much slower than the reaction of TF-bound factor VIIa; however, the amount of surface-bound factor VIIa may exceed the amount of TF-bound by orders of magnitude. This reaction is also limited by the amount of available surface. Unlike factor VIIa in a TF complex, factor VIIa without TF is not inhibited by TFPI.
Shibeko et al have attempted to bring some clarity to this question and to improve on earlier mathematical models of this process. It has previously been shown that zymogen factor VII decreases the rate of VIIa/TF activation of factor X by competing with factor VIIa for TF; increasing the concentration of factor VIIa can overcome this inhibition effect of plasma factor VII.4 Shibeko et al also observe this competition on both lipid vesicles and on cells. Further, they add to the possible significance of the TF-dependent mechanism by studying how binding of factor VIIa to TF might increase the rate of factor VII autoactivation. In part these studies involve some clever modifications to the existing mathematical models; these modifications account for TF density and for the fraction of factor VIIa bound to a surface (in a model that for all other reactions assumes infinite surface). Shibeko et al find that both models contribute to some extent to the enhanced thrombin generation in their biochemical and mathematical models but find that the TF-dependent mechanisms dominate. Their findings lower slightly the concentration of factor VIIa required to achieve a plateau because they find enhanced factor VII activation to be a major player.
The data of Shibeko et all add to our understanding of the biochemical mechanisms involved in factor X activation by factor VII(a) and TF. But in some sense these data still leave us with an intellectual problem regarding therapy. The authors note that it is not clear why increasing doses of factor VIIa would benefit a patient if the TF-dependent mechanism is predominant. And yet clinical studies looking at factor VIIa doses greater than 200 μg/kg suggest significantly increased efficacy in the high-dose group compared with the lower dose groups.7
It may be that future studies can further add to our understanding. Starting from the conclusion that the TF-dependent mechanism is dominant in vivo, it is possible to make 3 specific, testable hypotheses. One hypothesis is that factor VIIa molecules engineered to have increased TF-independent activity with the same level of TF-dependent activity will have similar dosing requirements to existing factor VIIa molecule in vivo. The second hypothesis, an extension of the first, is that a factor VIIa molecule engineered to have no TF binding would be ineffective in vivo regardless of the level of TF-independent activity. Finally, a factor VIIa molecule engineered to have no (or very reduced) activity (zymogen-like factor VIIa) unless bound to TF would be predicted to be effective in vivo with dosing requirements similar to existing factor VIIa molecules.
Understanding the in vivo mechanism has potential consequences for new therapies. If the dominant part of the mechanism of high-dose factor VIIa is mediated through TF, it suggests that therapies that enhance TF function, such as tissue factor pathway inhibitors, may be sufficient to correct hemophilia. By contrast, if the dominant part of the mechanism of high-dose factor VIIa is independent of TF, then factor VIIa molecules with enhanced TF-independent activity,8 that are specifically targeted to the platelet surface, or other molecules that have directed enhancements of platelet surface thrombin generation9 may be better future directions for new therapeutics.
Conflict-of-interest disclosure: The author has received institutional research grants from Novo Nordisk and Inspiration Biopharmaceuticals as well as honoraria from Novo Nordisk for speaking. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal