Factor VII circulates as a single chain inactive zymogen (10 nmol/L) and a trace (∼10-100 pmol/L) circulates as the 2-chain form, factor VIIa. Factor VII and factor VIIa were studied in a coagulation model using plasma concentrations of purified coagulation factors with reactions initiated with relipidated tissue factor (TF). Factor VII (10 nmol/L) extended the lag phase of thrombin generation initiated by 100 pmol/L factor VIIa and low TF. With the coagulation inhibitors TFPI and AT-III present, factor VII both extended the lag phase of the reaction and depressed the rate of thrombin generation. The inhibition of factor Xa generation by factor VII is consistent with its competition with factor VIIa for TF. Thrombin generation with TF concentrations >100 pmol/L was not inhibited by factor VII. At low tissue factor concentrations (<25 pmol/L) thrombin generation becomes sensitive to the absence of factor VIII. In the absence of factor VIII, factor VII significantly inhibits TF-initiated thrombin generation by 100 pmol/L factor VIIa. In this hemophilia A model, approximately 2 nmol/L factor VIIa is needed to overcome the inhibition of physiologic (10 nmol/L) factor VII. At 10 nmol/L, factor VIIa provided a thrombin generation response in the hemophilia model (0% factor VIII, 10 nmol/L factor VII) equivalent to that observed with normal plasma, (100% factor VIII, 10 nmol/L factor VII, 100 pmol/L factor VIIa). These results suggest that the therapeutic efficacy of factor VIIa in the medical treatment of hemophiliacs with inhibitors is, in part, based on overcoming the factor VII inhibitory effect.
The blood coagulation process starts by the contact of blood with tissue factor (TF), a transmembrane protein, that initiates the sequence of reactions that culminates in the generation of thrombin. TF is the essential cofactor for the serine protease–activated coagulation factor VII (factor VIIa).1,2 The factor VIIa·TF enzyme complex activates the zymogens factor X and factor IX by limited proteolysis.3 TF increases the catalytic efficiency of factor VIIa by a profound increase in kcat and a decrease in Km. Activated factor IX (factor IXa) combines with factor VIIIa on a membrane surface to form a secondary pathway to activate factor X. Activated factor X (factor Xa) associates with factor Va on a membrane surface to form prothrombinase, which converts prothrombin into thrombin, the key enzyme in hemostasis.
Most of the factor VII circulates as a single chain zymogen (10 nmol/L) and a trace (∼10-100 pmol/L) circulates in the active 2-chain form.4 The conversion of single chain factor VII (Mr = 50 000) to activated factor VIIa proceeds by a single cleavage after Arg152 to yield a Mr = 20 000 light chain derived from the NH2-terminal and a Mr = 30 000 heavy chain derived from the COOH-terminal, which are connected by a disulfide bond.5 Factor Xa, factor VIIa·TF, thrombin, factor IXa, and factor XIIa have been reported to activate factor VII.6-9 A comparison of the catalytic efficiencies of the potential physiologic factor VII activators showed that factor Xa, in association with phospholipids, possesses the highest potency to activate factor VII.10,11 The precise factor VIIa concentration in plasma and the pathway for the production of the basal levels of factor VIIa are still points of discussion.4,12Furthermore, factor VIIa is virtual inactive in the absence of TF and, in contrast to the other coagulation enzymes, stable in a plasma environment.4,12,13 The need for factor VII to circulate as an inactive zymogen is therefore not obvious. Factor VIIa possesses a higher potency in clotting assays compared with the zymogen factor VII, which indicates that the activation of factor VII is a limiting factor in thrombin generation. Furthermore, the treatment of hemophilia A or B patients with inhibitors by recombinant factor VIIa is highly effective,14 15 indicating the strong potential for factor VIIa–dependent enhancement of the thrombin generation process in vivo.
Zur et al16 showed that the activation of bovine factor IX by the bovine factor VIIa·TF complex is inhibited by physiologic levels of bovine zymogen factor VII. This inhibition of factor IX activation was only observed when factor VII could exert inhibition of factor VIIa through competitive binding to TF,16 ie, at limiting TF. Despite the growing body of biochemical knowledge concerning the rate of factor VII activation by the various proteases of the coagulation system, there has not been a clear description of the effects of zymogen factor VII on thrombin generation by the human coagulation factors.
This study describes the inhibitory effect of factor VII on factor VIIa·TF activity in the thrombin generation reaction, and evaluates the effects of zymogen factor VII and the enzyme factor VIIa in a reconstituted hemophilia A model.
Materials and methods
Reagents
Phosphatidylserine (PS) from bovine brain, phosphatidylcholine (PC) from egg yolk, and Hepes were purchased from Sigma. Spectrozyme TH and Spectrozyme Xa were purchased from American Diagnostica Inc, and Q-Sepharose FF was obtained from Pharmacia (Upsala, Sweden). All other reagents were of analytic grade.
Human coagulation factors X, IX, and prothrombin were isolated from 10 L fresh frozen plasma using the general methods of Bajaj et al17 and depleted from contaminants and traces of enzymes as described.18 Human coagulation factor VII was isolated essentially as described19 from the factor VII containing fractions eluted in the NaCl gradient of the DEAE-Sepharose chromatography, involved in the purification of the vitamin K–dependent clotting factors. Factor VII activity in the fractions was measured by incubating 25 μL of 1000-fold diluted samples in 20 mmol/L Tris, 150 mmol/L NaCl, pH 7.4 (TBS) containing 0.1% bovine serum albumin (BSA) with 50 μL of a solution containing 1/300 diluted rabbit thromboplastin (Thromboplastine-C, Dade, Baxter), 160 nmol/L factor X, and 10 mmol/L CaCl2 for 30 minutes. Generation of factor Xa was monitored using the chromogenic substrate Spectrozyme Xa. Factor VII eluted from the DEAE-Sepharose before and with some overlap with the first part of the C4BP/protein S peak. C4BP/protein S–containing fractions were not included in the factor VII pool. The factor VII pool was diluted 1.5 times with water containing 1 mmol/L benzamidine and applied to a 20-mL Q-Sepharose FF column previously equilibrated in 17 mmol/L Tris, 50 mmol/L NaCl, and 1 mmol/L benzamidine pH 7.4. After application of the pool, the column was washed with 100 mL equilibration buffer. Factor VII activity was eluted with 20 mmol/L CaCl2 in the same buffer. Fractions were collected in 1/10 of a volume of 0.4 mol/L EDTA pH 7.4. Factor VII containing fractions were pooled and precipitated by adding ammonium sulfate to 80% saturation. This solution was kept over night at 4°C, then spun in an SW-50 Rotor at 40 000 rpm, 4°C for 45 minutess. The protein pellet was resuspended in 50% glycerol and stored at −20°C. Before use in the experiment, factor VII was dialyzed extensively against TBS at 4°C. Factor VII prepared via this method appeared homogeneous on nonreduced and reduced SDS-PAGE and had a specific activity of 2000 U/mg measured in a single stage clotting assay.
Human factor V was isolated as described by Nesheim et al.20 Recombinant factor VIII and recombinant TF were provided as a gift from Dr Shu Len Liu, Hyland division, Baxter Healthcare Corp. Recombinant human coagulation factor VIIa was purchased from NOVO pharmaceuticals. Recombinant full length tissue factor pathway inhibitor (TFPI) was provided as a gift from Dr K. Johnson, Chiron Corp. Antithrombin III (AT-III) was purified from barium citrate–absorbed plasma by heparin-Sepharose chromatography according to the method described by Griffith et al.21AT-III was depleted from possible traces of heparin contamination using DEAE-cellulose chromatography as described.21
Coagulation factor activation experiments
Thrombin generation initiated by factor VIIa·TF was studied in a reconstituted coagulation model as described previously.18,22 23 TF was relipidated at the indicated concentrations in 400 μmol/L 75% phosphatidylcholine 25% phosphatidylserine vesicles, for 30 minutes at 37°C in 20 mmol/L Hepes, 150 mmol/L NaCl, 2 mmol/L CaCl2 pH 7.4 (Hepes/Ca++). Factor V and factor VIII were added to the relipidated TF mixture immediately before the reaction was started by the addition of a solution containing factor VIIa with or without factor VII and factor X, factor IX, and prothrombin. The zymogen solution was also prepared in Hepes/Ca++ and preheated at 37°C for 3 minutes before addition to the TF, factor V, and factor VIII mixture. When TFPI or AT-III were included, they were added to the factor X, factor IX, and prothrombin mixture.
The final concentrations of the proteins in the reaction, chosen to represent mean plasma values, were 160 nmol/L factor X, 90 nmol/L factor IX, 0.7 nmol/L factor VIII, 20 nmol/L factor V, 1.4 μmol/L prothrombin, 2.5 nmol/L TFPI, and 3.4 μmol/L AT-III. After starting the reaction, aliquots were withdrawn from the reaction mixture in time and quenched in 20 mmol/L EDTA/TBS pH 7.4 to assay for thrombin formation. Assays for thrombin activity were performed using the chromogenic substrate Spectrozyme TH. The hydrolysis of the substrate was monitored by the change in absorbance at 405 nm with a Molecular Devices Vmax spectrophotometer (Molecular Devices, Menlo Park, CA). Thrombin generation was calculated from a standard curve prepared by serial dilution of purified α-thrombin. When AT-III was added to the reaction mixture, samples were withdrawn in 20 mmol/L EDTA/TBS, containing 0.4 mmol/L Spectrozyme TH, and assayed immediately for thrombin activity as described before,18 to obtain accurate thrombin measurements in the presence of AT-III.
Results
Inhibition of TF-dependent thrombin generation by factor VII
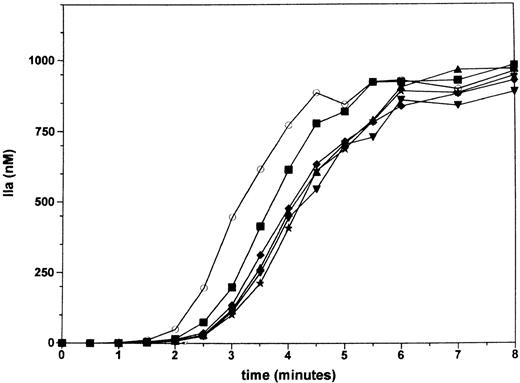
The effect of factor VII on the thrombin generation reaction was studied in a reconstituted procoagulant model using purified coagulation factors as described before.18,23 In Figure1 an experiment is displayed in which thrombin generation is initiated by adding a solution containing 100 pmol/L factor VIIa (the presumed plasma concentration of activated factor VII), factor X, factor IX, and prothrombin to a solution containing 1.25 pmol/L relipidated tissue factor, factor V, and factor VIII. Thrombin generation by 100 pmol/L factor VIIa and 1.25 pmol/L TF occurs after a 90-second lag phase. This lag, or initiation, phase involves the binding of factor VIIa to TF, the generation of small amounts of factor Xa and IXa, formation of traces of thrombin, and the near quantitative activation of the cofactors factor V and factor VIII. After the lag phase, thrombin generation becomes explosive during a propagation phase via increased factor Xa generation by factor IXa–factor VIIIa activity.18 Thrombin generation reaches a maximum of 400 nmol/L/min at 180 seconds, resulting in the quantitative conversion of prothrombin at 4 minutes. No thrombin generation occurred when either factor VIIa or TF were omitted from the reaction. The addition of factor VII to the mixture containing 100 pmol/L factor VIIa resulted in a concentration-dependent increase in the lag phase from 0.5 to 1 nmol/L factor VII. A further increase in the lag phase was not observed when the concentration of factor VII was raised to 10 nmol/L, the approximate plasma concentration of the zymogen. No effect of factor VII was observed under these conditions on the maximal rate of thrombin generation during the propagation phase.
Inhibition of TF-dependent thrombin generation by factor VII.
Thrombin generation was initiated by mixing a solution that contained relipidated TF, factor V, and factor VIII with a solution that contained factor VIIa, factor X, factor IX, prothrombin, and various concentrations of zymogen factor VII. Final concentrations in the reaction were 1.25 pmol/L TF, 100 pmol/L factor VIIa, and plasma concentrations of the other coagulation factors, except for factor VII, which is at 0.5 (▪), 1 (♦), 2.5 (▴), 5 (*), or 10 nmol/L (▾) and absent in the control reaction (○). Factor VII inhibits only the initial phase of thrombin generation in the absence of other coagulation inhibitors.
Inhibition of TF-dependent thrombin generation by factor VII.
Thrombin generation was initiated by mixing a solution that contained relipidated TF, factor V, and factor VIII with a solution that contained factor VIIa, factor X, factor IX, prothrombin, and various concentrations of zymogen factor VII. Final concentrations in the reaction were 1.25 pmol/L TF, 100 pmol/L factor VIIa, and plasma concentrations of the other coagulation factors, except for factor VII, which is at 0.5 (▪), 1 (♦), 2.5 (▴), 5 (*), or 10 nmol/L (▾) and absent in the control reaction (○). Factor VII inhibits only the initial phase of thrombin generation in the absence of other coagulation inhibitors.
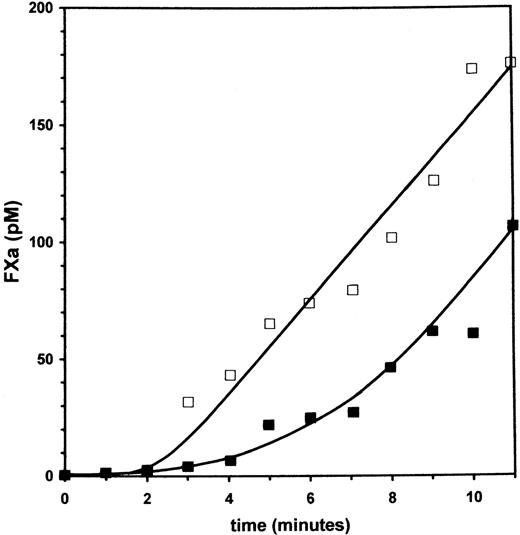
Inhibition of tissue factor dependent factor Xa generation by factor VII
In Figure 2, an experiment is displayed that shows the effect of factor VII on the generation of factor Xa by factor VIIa and TF. The reaction was initiated by addition of a mixture containing factor X and factor VIIa, with or without zymogen factor VII, to relipidated TF. Final concentrations were 100 pmol/L factor VIIa, factor VII when added 10 nmol/L, 1.25 pmol/L tissue factor, and 500 nmol/L factor X. Factor Xa generation was measured using the chromogenic substrate Spectrozyme Xa as described under Methods. Factor Xa generation in the absence of the zymogen (open squares), starts with a delay of 2 minutes, which reflects the formation of factor VIIa·TF complexes and proceeds after 2 minutes with an apparent almost instantaneous maximal rate of 19 pmol/L/min Xa. In the presence of 10 nmol/L of the zymogen factor VII (filled squares), factor Xa generation starts with a delay of 4 minutes and results in a similar rate of factor Xa generation, but only after 6 minutes in the reaction. This profile of factor Xa generation is consistent with initial competition of factor VII and factor VIIa for TF binding. At 1.25 pmol/L TF the maximal factor Xa generation is obviously limited by the tissue factor concentration in this experiment. From this, it follows that the observed identical maximal rate in the incubation with factor VII is most likely the result of feedback activation of factor VII by factor Xa, and saturation of TF with factor VIIa.
Inhibition of TF-dependent factor Xa generation by factor VII.
The effect of zymogen factor VII on factor Xa generation by TF and the physiologic trace of factor VIIa was investigated by mixing a solution containing a limited amount of relipidated TF with a solution containing factor X and factor VIIa without (□) or with (▪) 10 nmol/L factor VII. Final concentrations in the reaction were as follows: 1.25 pmol/L TF, 100 pmol/L factor VIIa, and 500 nmol/L factor X. The initial inhibition and final identical rate of factor X activation in the reaction with factor VII is consistent with competitive binding to TF and feedback activation of the zymogen by factor Xa formed in the reaction.
Inhibition of TF-dependent factor Xa generation by factor VII.
The effect of zymogen factor VII on factor Xa generation by TF and the physiologic trace of factor VIIa was investigated by mixing a solution containing a limited amount of relipidated TF with a solution containing factor X and factor VIIa without (□) or with (▪) 10 nmol/L factor VII. Final concentrations in the reaction were as follows: 1.25 pmol/L TF, 100 pmol/L factor VIIa, and 500 nmol/L factor X. The initial inhibition and final identical rate of factor X activation in the reaction with factor VII is consistent with competitive binding to TF and feedback activation of the zymogen by factor Xa formed in the reaction.
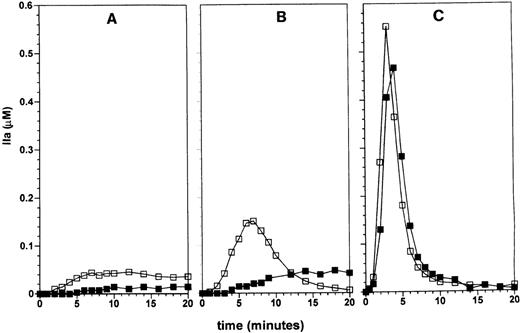
Effect of factor VII on thrombin generation in the presence of TFPI and AT-III
We had previously shown that the coagulation inhibitors regulate thrombin generation in a synergistic fashion.18 We therefore investigated the influence of physiologic amounts of factor VII on thrombin generation in the presence of the stoichiometric inhibitors TFPI and AT-III, known regulators of TF, factor Xa, factor IXa, and thrombin activity. Figure 3A shows thrombin generation by 100 pmol/L factor VIIa and 10 pmol/L TF in the presence of 2.5 nmol/L TFPI and 3.4 μmol/L AT-III (open squares). Thrombin generation is observed after a lag period of 1 minute and reaches a value of 45 nmol/L at 7 minutes, which remains stable up to 20 minutes. The stable level of thrombin activity is a result a balance of the opposing rates of thrombin formation and its inactivation by AT-III. In the presence of 10 nmol/L factor VII, the lag time was increased by 3 minutes to 4 minutes (filled squares) and a maximal level of 18 nmol/L thrombin was reached at 10 minutes, which was sustained up to 20 minutes in the reaction. At 20 pmol/L TF, thrombin generation starts after 30 seconds with a maximal rate of generation of 45 nmol/L/min and reaches a level of 150 nmol/L at 7 minutes, after which thrombin activity declines to 5 nmol/L at 20 minutes (Figure 3B). In the presence of factor VII, thrombin generation by 20 pmol/L TF starts after an increased lag period of 3 minutes with a maximal rate of 4 nmol/L/min reaching a maximal level of 45 nmol/L at 14 minutes, which remained stable up to 20 minutes. At 100 pmol/L TF, thrombin generation becomes explosive after 30 seconds in the presence of 100 pmol/L factor VIIa with a maximal rate of 250 nmol/L/min and reaches an optimal value at 3 minutes (Figure 3C). Thrombin activity declined to 20 nmol/L at 10 minutes in the reaction and residual thrombin activity is further inhibited in time. This profile reflects total prothrombin consumption and subsequent inhibition of active thrombin by AT-III. The reaction initiated by 100 pmol/L factor VIIa and 100 pmol/L TF in the presence of factor VII is not significantly delayed by 10 nmol/L factor VII and shows the same profile as the reaction with factor VIIa alone.
Effect of factor VII on thrombin generation in the presence of TFPI and AT-III.
The effect of zymogen factor VII on TF-dependent thrombin generation was investigated as described in Figure 1, except that the natural coagulation inhibitors TFPI and AT-III were present at their respective plasma concentrations in the final reaction mixture. Reactions were initiated by 10 (panel A), 20 (panel B), or 100 (panel C) pmol/L TF in the presence of 100 pmol/L factor VIIa in the absence (□) or presence (▪) of 10 nmol/L factor VII. In the presence of TFPI and AT-III the inhibitory effect of factor VII on TF-dependent thrombin generation is not only restricted to the initial part of the reaction, but also decreases the maximal observed thrombin generation in reactions initiated by TF concentrations of ≤ 20 pmol/L. At TF concentrations of 100 pmol/L and higher thrombin generation occurs explosively, regardless of the presence of inhibitors or zymogen factor VII.
Effect of factor VII on thrombin generation in the presence of TFPI and AT-III.
The effect of zymogen factor VII on TF-dependent thrombin generation was investigated as described in Figure 1, except that the natural coagulation inhibitors TFPI and AT-III were present at their respective plasma concentrations in the final reaction mixture. Reactions were initiated by 10 (panel A), 20 (panel B), or 100 (panel C) pmol/L TF in the presence of 100 pmol/L factor VIIa in the absence (□) or presence (▪) of 10 nmol/L factor VII. In the presence of TFPI and AT-III the inhibitory effect of factor VII on TF-dependent thrombin generation is not only restricted to the initial part of the reaction, but also decreases the maximal observed thrombin generation in reactions initiated by TF concentrations of ≤ 20 pmol/L. At TF concentrations of 100 pmol/L and higher thrombin generation occurs explosively, regardless of the presence of inhibitors or zymogen factor VII.
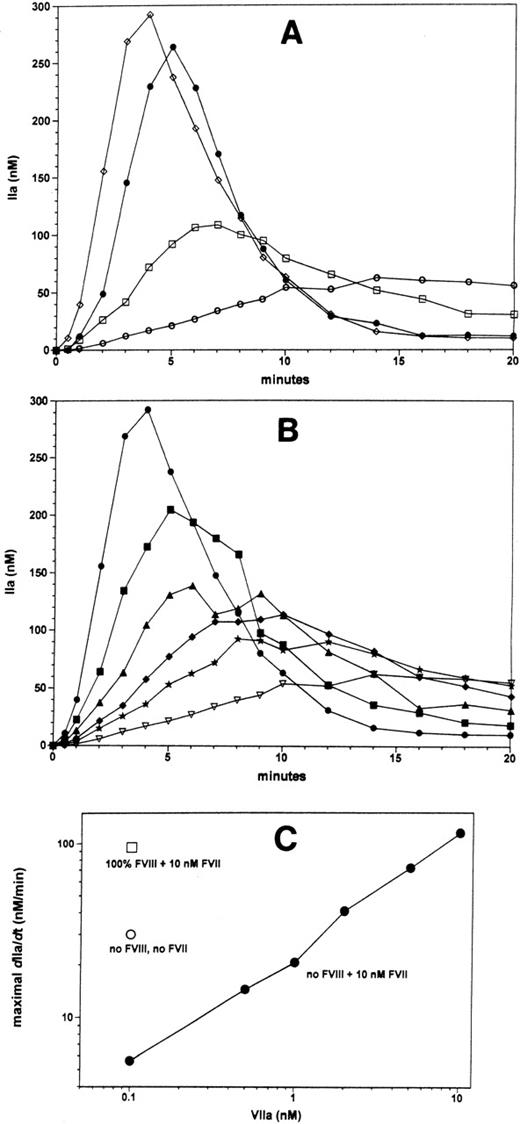
Effects of factor VII and factor VIIa in the absence of factor VIII
The very significant inhibitory effect of factor VII on thrombin generation initiated by physiologic traces factor VIIa and limiting TF may explain why treatment of hemophiliacs with factor VIIa is so effective. We performed experiments that basically recapitulate the treatment of hemophilia A with recombinant factor VIIa in our in vitro thrombin generation model. The effects of factor VII and factor VIIa on thrombin formation in the hemophilia A situation (no factor VIII) are shown in Figure 4A. Reactions were initiated using TF concentrations that produce factor VIII–dependent thrombin generation in the presence of AT-III and TFPI. In the presence of the physiologic factor VII (10 nmol/L) and factor VIIa (100 pmol/L) concentrations, thrombin generation occurs very slowly in the absence of factor VIII (open circles) compared with the reaction with factor VIII (filled circles). Factor VII also inhibits thrombin generation in reactions without factor VIII, as omission of factor VII in the hemophilia A situation results in a 5-fold enhancement of the maximal rate of thrombin generation (open squares). Factor VIIa titration in the hemophilia A situation in the model in which thrombin generation is inhibited by 10 nmol/L zymogen factor VII (Figure 4B) results in concentration-dependent increases in the maximal rate of thrombin formation and concentration-dependent increases of the maximum levels of thrombin formed. Figure 4C summarizes the data by plotting the maximum rate of thrombin generation (dIIa/dt), indicative for the amount of prothrombinase activity formed in the reaction. Approximately 2 nmol/L factor VIIa is needed to overcome the inhibitory effect of factor VII on thrombin generation in the absence of factor VIII (Figure4B and C). By the time 10 nmol/L factor VIIa is attained, a normal thrombin generation profile is restored in the absence of factor VIII (compare Figure 4A open diamonds and filled circles and Figure 4C open squares and filled circles). The factor VIIa levels needed to normalize thrombin generation in the hemophilia A situation in the presented model are consistent with the levels of factor VIIa that will provide normal hemostasis in patients with a factor VIII inhibitor.15
Effects of factor VII and factor VIIa in the absence of factor VIII.
The effects of factor VII and factor VIIa under hemophilia A conditions (without factor VIII) were studied on TF-dependent (5 small pMTF) thrombin generation in the presence of TFPI and AT-III as described in Figure 3. Panel A shows thrombin generation curves in the presence of the physiologic ratio of factor VIIa:factor VII (100 pmol/L:10 nmol/L) in the absence (○) or presence (•) of factor VIII. Omission of factor VII from the reaction in the absence of factor VIII (panel A, □) results in a considerable increase in the rate of thrombin generation. Addition of 10 nmol/L factor VIIa to the reaction with factor VII and absence of factor VIII (panel A, ⋄) results in a normalization of the thrombin generation profile. Panel B displays thrombin generation curves, from the contemporaneous experiment shown in panel A, which were performed in the absence of factor VIII and presence of 10 nmol/L factor VII with a range of factor VIIa concentrations, namely, 0.1 (▿), 0.5 (*), 1 (♦), 2 (▴), 0.5 (▪), and 10 nmol/L (•). Panel C shows the maximal observed rates thrombin generation in curves shown in panel A as a function of the factor VIIa concentration; absence and presence of factor VIII and factor VII are indicated in the figure in panel C.
Effects of factor VII and factor VIIa in the absence of factor VIII.
The effects of factor VII and factor VIIa under hemophilia A conditions (without factor VIII) were studied on TF-dependent (5 small pMTF) thrombin generation in the presence of TFPI and AT-III as described in Figure 3. Panel A shows thrombin generation curves in the presence of the physiologic ratio of factor VIIa:factor VII (100 pmol/L:10 nmol/L) in the absence (○) or presence (•) of factor VIII. Omission of factor VII from the reaction in the absence of factor VIII (panel A, □) results in a considerable increase in the rate of thrombin generation. Addition of 10 nmol/L factor VIIa to the reaction with factor VII and absence of factor VIII (panel A, ⋄) results in a normalization of the thrombin generation profile. Panel B displays thrombin generation curves, from the contemporaneous experiment shown in panel A, which were performed in the absence of factor VIII and presence of 10 nmol/L factor VII with a range of factor VIIa concentrations, namely, 0.1 (▿), 0.5 (*), 1 (♦), 2 (▴), 0.5 (▪), and 10 nmol/L (•). Panel C shows the maximal observed rates thrombin generation in curves shown in panel A as a function of the factor VIIa concentration; absence and presence of factor VIII and factor VII are indicated in the figure in panel C.
Discussion
In this study, we find that the zymogen factor VII functions as an inhibitor of thrombin generation initiated by the physiologic trace amount of active factor VIIa and low concentrations of TF. In a highly reactive system with the procoagulant potential formed by endogenous activation of purified coagulation factors X, IX, VII, V, VII, and prothrombin on phospholipid, we show an inhibitory effect by factor VII on TF-initiated thrombin generation. The effect is observed at a factor VIIa-to-factor VII ratio 1:10 (100 pmol/L:1 nmol/L) under conditions in which the TF concentration is limiting factor Xa and thrombin formation. Direct measurement of factor Xa generation in an isolated reaction with TF, factor VIIa, and factor X showed that factor VII delayed factor Xa generation by 100 pmol/L factor VIIa and 1.25 pmol/L TF, but reached the same rate of factor Xa generation in time. The inhibitory kinetics of factor Xa generation by factor VII are consistent with the initial formation of primarily factor VII·TF complexes caused by the molar excess of factor VII at the start of the reaction. Subsequently, the traces of factor Xa initially formed generate the full potential of factor VIIa·TF activity by feedback activation of factor VII. Our results with the human proteins are in perfect agreement with the report by Zur et al16 of inhibition of factor VIIa·TF activity via competitive binding by zymogen factor VII in the bovine system. Similarly, inhibition of factor VIIa·TF activity by competitive binding to TF of active site blocked factor VIIa (FVIIai) is presently used as an antithrombotic drug.24 Our current data, however, are the first to show that physiologic levels of zymogen factor VII will actually dampen thrombin generation by the trace plasma levels of factor VIIa considerably at low initiating TF. In the presence of the stoichiometric coagulation inhibitors TFPI and AT-III, addition of factor VII resulted in a drastic delay in thrombin generation and subsequently significant lower rates of thrombin generation. This inhibitory action of factor VII on the TF-initiated coagulation process might be of great importance in the regulation of the hemostatic response. Indications for inhibition of the in vivo thrombin generation process by zymogen factor VII were put forward by Bauer et al25 based on treatment of factor VII–deficient patients with factor VIIa. Namely, infusion of a bolus recombinant factor VIIa in a factor VII–deficient patient with < 1% factor VII antigen and coagulant activity, resulted in substantial fibrinopeptide A (FPA) generation. Because no FPA generation was observed on factor VIIa administration in a factor VII–deficient patient with 16% antigen and 3% coagulant activity, Bauer and colleagues concluded that the endogenous factor VII present in this patient inhibited thrombin generation. The hypothesis that zymogen factor VII inhibits thrombin generation was, however, never substantiated. The current in vitro data strongly support the role for factor VII as a physiologic downregulator of thrombin generation. The level of 16% factor VII antigen (1.6 nmol/L) in the patient who did not respond with FPA generation on factor VIIa infusion corresponds with our observation that factor VII at concentrations ≥ 1 nmol/L can inhibit thrombin generation significantly. In a previous study, we found that factor VII activation only occurs at a late stage in our reconstituted thrombin generation model initiated with limited TF.23 This late factor VII activation was found to be mainly dependent on the high levels of thrombin formed in the absence of AT-III.23 Consistent with this, Rao et al26reported only limited factor VII activation, evaluated via monitoring cleavage of radiolabeled factor VII, in plasma clotted with diluted tissue factor. The latter indicates the relative slow kinetics of factor VII activation, which consequently may result in a protracted inhibition of TF by zymogen factor VII.
Activated factor VIIa is more potent in clotting assays compared with the zymogen.27 28 Factor VII evaluation by clotting assay measures both the activated form and the zymogen form, and the ratio of the potency of either factor VIIa or factor VII in these assays is dependent on the materials used and how these assays are performed. The general-applied clotting assay to estimate the level of factor VII in plasma samples use relatively high concentrations of TF. This will not allow the monitoring of the inhibitory effect of factor VII on the clotting reaction by lack of competition by factor VIIa and factor VII for the abundant TF in these assays.
The inhibiting effect of factor VII occurs in reactions that are initiated with TF concentrations of ≤ 20 pmol/L. It is also at this concentration of tissue factor that the thrombin generation pattern changes from an explosive burst of thrombin to a tightly regulated appearance of thrombin in the presence of combinations of the coagulation inhibitors.18,29 Furthermore, the reaction becomes heavily dependent on factor IXa–factor VIIIa activity at these concentrations of TF in our reconstituted model18 and in whole blood.30 Hemophilia A patients with inhibitors can be treated by intravenous administration of bolus or continuous infusion of factor VIIa, resulting in plasma concentrations of ∼ 10 to 20 U/mL.14,15,31,32 The plasma concentration of factor VII antigen doubled in the initial study reported by Hedner et al,14 indicating equimolar concentrations of factor VIIa and factor VII in the patient's plasma. Recent studies recommend to maintain a plasma level of > 6 or 10 U/mL in hemophilia patients with inhibitors during episodes of bleeding or surgical treatment.31,32 With the specific activity of 50.000 U/mg of the recombinant factor VIIa product of Novo Nordisk, this means a plasma concentration of 120 to 200 ng/mL, which corresponds to 2.4 to 4 nmol/L factor VIIa.14 15 The apparent clinical enhancement of the clotting reaction by factor VIIa is not completely understood. In our in vitro model, however, we show that ∼ 2 nmol/L factor VIIa is needed to overcome the inhibitory effect of factor VII on thrombin generation in the hemophilia situation, and that 10 nmol/L factor VIIa normalizes the thrombin generation profile to the reaction with normal plasma levels of factor VIII, factor VII, and factor VIIa. Thus, our results are in excellent agreement with the recommended target plasma levels of factor VIIa in the treatment of hemophiliacs with inhibitors. Furthermore, our results show that the therapeutic mechanism of factor VIIa treatment of hemophilia A is, at least in part, the result of overcoming the inhibition of tissue factor activity by the zymogen factor VII.
Others have put forward the possibility that factor VIIa may provide hemostasis in hemophilia models via a platelet-dependent and TF-independent mechanism.33 This is, however, contrasted by the observation that the increased levels of the activation fragments of factor X and prothrombin after infusion of recombinant factor VIIa in chimpanzees is completely dependent on tissue factor activity.34 Consistently, inhibition of TFPI restores the hemostatic potential in a hemophilia A rabbit model.35 The inhibitory potential of TFPI on factor VIIa·TF activity causes the need for additional factor Xa generation via the factor IXa · factor VIIIa complex.18,36 Because thrombin generation in our model is dampened by factor VII to a large extent in the presence of TFPI, it could also be hypothesized that the use of factor VIIa ensures a burst of the full potential of exposed TF before it is inhibited by TFPI. The inhibition of traces of factor VIIa activity by the bulk factor VII zymogen is probably the earliest regulating step in the process leading to thrombin generation. The extension by factor VII of the lag phase of TF–initiated thrombin generation could have been expected, since several studies have shown that the lag phase is dependent on the concentration of factor VIIa·TF activity, the latter complex has to build up in the presence of the zymogen. The above mentioned regulation of the coagulation process involves the initiation phase of the thrombin generation reaction. In this phase, factor Va is formed by initial traces of thrombin.23 Factor Va is not only important as cofactor for factor Xa but will also shield the enzyme from inhibition by TFPI and AT-III.37 38 From our in vitro experiments, it appears that the protraction of the initial processes of thrombin generation by the zymogen factor VII results in significant inhibition of the coagulation process.
In conclusion, we describe the inhibition of tissue factor–initiated thrombin generation by the zymogen factor VII. This observation provides a rational for the treatment of patients with bleeding disorders with activated factor VIIa.
Supported by a TALENT-stipendium of the Netherlands Organization of Scientific Research to C.v.V. and by HL-46703 from the National Institutes of Health to K.G.M.
C.v.V. is currently at the Department of Surgery, University of Maastricht, Maastricht, Netherlands. N.J.G. is currently at the School of Medicine, New England Medical Center, Tufts University, Boston, MA.
Reprints:K.G. Mann, Department of Biochemistry, College of Medicine, University of Vermont, Burlington, VT 05405.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal