Inhibitory antibody formation is a major complication of factor VIII replacement therapy in patients with hemophilia A. To better understand the pathogenesis of this immunologic reaction, we evaluated the role of T-cell costimulatory signals for antifactor VIII antibody formation in a murine model of hemophilia A. Repeated intravenous injections of factor VIII in these factor VIII–deficient mice induced an antifactor VIII inhibitor antibody response. This response was shown to be T-cell dependent by its absence in hemophilic mice also deficient for the T-cell costimulatory ligand B7-2. In separate experiments, injection of murine CTLA4-Ig completely blocked the primary response to factor VIII in hemophilic mice with intact B7 function. This reagent also prevented or diminished further increases in antifactor VIII when given to hemophilic mice with low antifactor VIII antibody titers. These studies suggest that strategies targeting the B7-CD28 pathway are potential therapies to prevent and treat inhibitory antifactor VIII antibodies. Moreover, because the development of antibodies to replaced proteins may limit the success of many human gene therapy approaches, our results may be broadly applicable.
Hemophilia A is an X-linked bleeding disorder caused by the deficiency in plasma of the glycoprotein factor VIII.1However, approximately one-third of patients with severe hemophilia A develop an antifactor VIII inhibitor following factor VIII replacement therapy.2 Current strategies to blunt the antibody response in these patients have only been marginally successful. Moreover, the development of antibodies to replaced proteins is a critical problem that needs to be solved if gene therapy is to be successful in the treatment of hemophilias and other deficiency diseases.3 4
We have used a mouse model of hemophilia A to evaluate new methods for the prevention and treatment of inhibitor formation. Hemophilia A mice, generated by targeted disruption of exon 16 (E-16) of the factor VIII gene, have no detectable factor VIII activity in their plasma5 and are similar in this way to patients with severe hemophilia A. As expected, hemophilia A mice have in vivo signs of a coagulation pathway defect with fatal bleeding if tails are cut without use of hemostatic measures, and they develop subcutaneous and intramuscular bleeding after handling or temporary immobilization.6-8
Intravenous infusions of 0.2 μg human factor VIII, a dose equivalent on a weight basis to that given to hemophilia A patients, resulted in minimal or no antibody response in these hemophilia A mice after a single injection, but repeat infusions led to high titer inhibitory antifactor VIII.6 However, before antibodies were detected, a factor VIII–specific T-cell proliferative response was detected 3 days after the first exposure to human factor VIII. This suggested that the antibody response to factor VIII is T-cell dependent, so that blockade of T-cell activation might prevent inhibitor antibody formation in hemophilia A mice.
Optimal T-cell activation requires signaling through the antigen-specific T-cell receptor (TCR) by its engagement with peptide major histocompatibility complex (MHC) complexes on antigen-presenting cells. This is completed in combination with costimulatory signals typically delivered through the T-cell surface glycoprotein, CD28.9 CD28 interactions with B7-1 (CD80) and B7-2 (CD86), costimulatory ligands on antigen-presenting cells, are essential for initiating antigen-specific T-cell responses, up-regulating cytokine expression, and promoting T-cell expansion and differentiation.9
CTLA4 is a second high-affinity T-cell receptor for both the B7-1 and B7-2 ligands.9 CTLA4 is a down-regulatory molecule in T-cell activation, as demonstrated by the lymphoproliferative phenotype of the CTLA4-deficient mouse strain.10,11CTLA4-immunoglobulin (CTLA4-Ig), a soluble fusion protein in which the extracellular domain of CTLA4 is fused to the heavy chain constant regions 2 and 3 (CH2-CH3) tail of IgG1, has been shown to be an effective reagent for blocking CD28-B7 interactions in vivo because CTLA4-Ig binds to B7-1 and B7-2 ligands and blocks B7 interactions with both CD28 and CTLA4.12 Blockade of the CD28 signaling pathway has been used successfully to prevent T-cell dependent responses in many animal models of autoimmunity and transplantation.9,13,14 Very recently, the first successful clinical applications of CTLA4-Ig–mediated blockade of T-cell costimulation have been reported.15 16
We report here evidence that the development of inhibitory antibodies to factor VIII is T-cell dependent. Moreover, murine CTLA4-Ig (mCTLA4-Ig) blockade is an effective means of preventing the induction of an antifactor VIII response and suppressing secondary antifactor VIII responses.
Materials and methods
Animals
The characteristics of the E-16 strain of hemophilic mice have been reported.5 17 Adult male and homozygous E-16 female mice, aged 10-20 weeks, were used for these studies. Blood samples were obtained by orbital venous plexus bleeding, and the serum was separated by centrifugation at 600g for 3 minutes. The serum samples were stored at 20°C until the samples were assayed. To avoid severe bleeding and death of animals, ear tags were not used to identify the mice in some experiments. For this reason, Figure 1does not indicate sequential data for individual mice.
The role of B7-1 and B7-2 antigens in the antifactor VIII antibody response.
Hemophilia A/B7-1−/− (○) and hemophilia A/B7-2−/− (•) mice were injected intravenously with 0.2 μg factor VIII at 2-week intervals. Serum samples for antifactor VIII assay were obtained 12 days after the second and sixth factor VIII injections.
The role of B7-1 and B7-2 antigens in the antifactor VIII antibody response.
Hemophilia A/B7-1−/− (○) and hemophilia A/B7-2−/− (•) mice were injected intravenously with 0.2 μg factor VIII at 2-week intervals. Serum samples for antifactor VIII assay were obtained 12 days after the second and sixth factor VIII injections.
Generation of E-16/B7-1 and E-16/B7-2 double knockout mice was accomplished by cross breeding of E-16 with B7-1 and B7-2 knockout mice.18 Homozygous E-16/B7-1 and E-16/B7-2 double knockout mice were identified by genotype determination.5,18 Reduced factor VIII activity was verified using a chromogenic bioassay (Coatest; Chromogenix, MoIndal, Sweden).17 The factor VIII activity was less then 1% in both E-16/B7-1−/− and E-16/B7-2−/− mice.
Antigens
Recombinant human factor VIII (Baxter Healthcare, Hyland Division; Glendale, CA) was used in the study.
Murine CTLA4-Ig
A mCTLA4-Ig complementary DNA (cDNA) expression plasmid was prepared by ligation of the leader and extracellular domains of mCTLA4 to the hinge, the CH2 and CH3 domains of IgHg2a that had been mutated to remove effector functions, as described in Streurer.19 The insert was cloned into the expression vector pED and stably transfected into Chinese hamster ovary cells as previously described.20 Concentrated conditioned media was loaded onto a chromatography column (Protein A Sepharose Fast Flow; Amersham Pharmacia Biotech, Piscataway, NJ). The column was washed with phosphate-buffered saline (PBS, pH 7.1), and the mCTLA4-Ig was eluted with 20 mmol/L citrate (pH 3.0). The peak pool was neutralized with 1 mol/L tris(hydroxymethyl) aminomethane (Tris, pH 8.0) to a final pH of 7.5 and formulated into PBS (pH 7.1) using an Amicon-stirred cell with a YM30 membrane (Millipore Corp, Bedford, MA). The mCTLA4-Ig was depyrogenated using a chromatography column (Poros PI; Perceptive Biosystems, Framingham, MA), and the product was eluted from the column in a linear sodium chloride (NaCl) gradient from 0 to 1 mol/L NaCl in 25 mmol/L Tris (pH 7.5). The mCTLA4-Ig was then formulated into PBS (pH 7.1) using an Amicon-stirred cell with a YM30 membrane.
Antibody measurements
The antifactor VIII titer was determined by enzyme-linked immunosorbent assay (ELISA).6 The ELISA tests were carried out using microtiter wells coated with 0.8 μg/mL recombinant human factor VIII in 0.05 mol/L carbonate-bicarbonate (pH 9). After mouse plasma samples were incubated in the wells at 4°C overnight and then washed, alkaline-phosphatase–conjugated goat antimouse IgG (Southern Biotechnology Associates, Birmingham, AL) was added for 2 hours at room temperature. After washing, we added 2 mg/mL P-nitrophenyl phosphate (Sigma, St. Louis, MO) in 100 mmol/L glycine, 1 mmol/L magnesium dichloride (MgCl2), and 2 mmol/L zinc dichloride (ZnCl2) (pH 10.4). The absorbance was read at 410 nm using an automated micro titer plate ELISA reader. The concentration of antifactor VIII antibody was estimated from a standard curve obtained using a factor VIII monoclonal antibody (mAb) that binds to the A2 domain, mAb 413.21 The titer was calculated from points that fell on the linear portion of the assay standard curve. Antifactor VIII inhibitor titers in Bethesda units (BU) were measured by the Bethesda assay.22
T-cell proliferation assays
The spleen was used as the source of T cells for proliferation assays. Spleen cells were then cultured (5 × 105cells/well) in 96-well flat-bottom plates. Varying amounts of recombinant factor VIII were added to the culture medium consisting of complete RPMI 1640 (Gibco BRL, Rockville, MD) containing 0.5% hemophilic mouse serum. After 72 hours of culture at 37°C, we added 0.074 MBq (2 μCi) 3H-thymidine/well (ICN Pharmaceuticals, Irvine, CA). The cultures were harvested 16 hours later (Matrix 9600; Packard, Meriden, CT). Data are expressed as the mean for triplicate wells of the cpm incorporated into insoluble DNA.
Results
Primary immune response to factor VIII
The roles of B7-1 and B7-2 costimulatory ligands present on antigen-presenting cells were evaluated to determine if the T-cell CD28 signaling pathway is essential for the development of inhibitory antibodies to factor VIII. To do this we crossed hemophilia A mice with B7-1−/− and B7-2−/−knockout mice.18,23 Mice deficient in both factor VIII and either B7-1 or B7-2 ligands were selected by genotype analysis. The hemophilia A/B7-1−/− and hemophilia A/B7-2−/−mice were then injected intravenously with 0.2 μg human factor VIII at 2-week intervals. After 4 injections, all 9 hemophilia A/B7-1−/−mice had developed antifactor VIII, with ELISA antibody titers greater than 350 μg/mL and a mean inhibitor level of 712 BU (Figure 1). These are values similar to those for otherwise normal hemophilia A mice injected with factor VIII.6 In contrast, none of the 8 hemophilia A/B7-2−/−mice had detectable antifactor VIII.
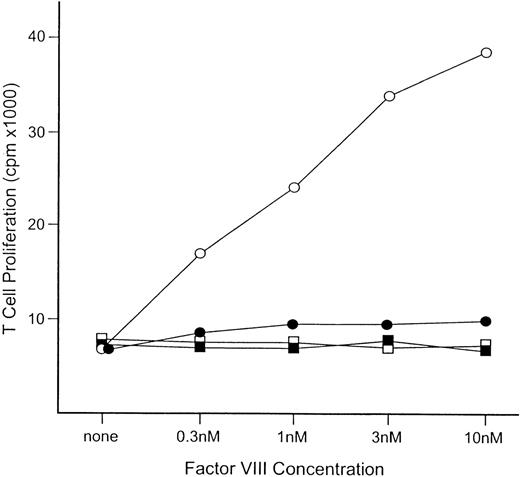
To evaluate the T-cell response of these B7-1– and B7-2–deficient hemophilia A mice, spleen cells were obtained 3 days after a fifth intravenous injection of factor VIII. The T-cell proliferative activity determined by 3H-thymidine incorporation showed a factor VIII dose-dependent response for cells from the hemophilia A/B7-1−/−mice (Figure2). In contrast, T-cell response was not detected at any factor VIII level for spleen cells from hemophilia A/B7-2−/−mice. Thus, the B7-2 ligand is essential for the development of an immune response to factor VIII injected intravenously, and antifactor VIII formation is prevented if it is missing.
T-cell response to factor VIII for hemophilia A/B7-1−/− and hemophilia A/B7-2−/−mice.
Spleen cells were obtained 3 days after the fifth intravenous injection of human factor VIII. Pooled spleen cells from 3 mice were used to establish the proliferation data. The open and closed squares are for cells from untreated hemophilia A/B7-1−/− and B7-2−/−mice. Five intravenous injections of factor VIII were given to hemophilia A/B7-1−/−mice (○) and hemophilia A/B7-2−/−mice (•). The concentration of factor VIII in the cultures is indicated on the horizontal axis.
T-cell response to factor VIII for hemophilia A/B7-1−/− and hemophilia A/B7-2−/−mice.
Spleen cells were obtained 3 days after the fifth intravenous injection of human factor VIII. Pooled spleen cells from 3 mice were used to establish the proliferation data. The open and closed squares are for cells from untreated hemophilia A/B7-1−/− and B7-2−/−mice. Five intravenous injections of factor VIII were given to hemophilia A/B7-1−/−mice (○) and hemophilia A/B7-2−/−mice (•). The concentration of factor VIII in the cultures is indicated on the horizontal axis.
Blocked induction of an antifactor VIII response by mCTLA4-Ig
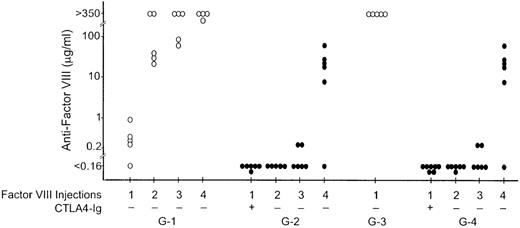
As mCTLA4-Ig has been shown to be an effective reagent for blocking CD28-B7-2 interactions in vivo,12 we tested the effectiveness of this blockade of the CD28 signaling pathway in preventing antifactor VIII inhibitor antibody formation. Antifactor VIII inhibitory antibodies were induced in control hemophilia A mice by repeated intravenous injections of 1 μg recombinant human factor VIII at 3-week intervals (group G-1, Figure 3). Antifactor VIII was detected in 4 of the 5 mice 20 days after the first injection, and all control mice developed high-titer antifactor VIII after receiving 2 to 4 injections. The mean inhibitor level after 4 injections was 1860 BU. Antifactor VIII antibody formation was markedly suppressed in mice injected intraperitoneally with 250 μg of murine CTLA4-Ig on the day before and the day after the first factor VIII injection (group G-2, Figure 3), even though there was no further mCTLA4-Ig given with 3 subsequent factor VIII injections on days 23, 44, and 66. Antifactor VIII was not detectable in any group G-2 mice after the first or second factor VIII injection. Three weeks after the third injection of factor VIII, a weak immune response was detected in 2 of the 6 mice in group G-2.
The effect of mCTLA4-Ig on antifactor VIII antibody formation.
Four groups of hemophilia A mice were injected with recombinant human factor VIII on days 0, 23, 44, and 66 (initially 1 μg intravenously and then 0.2 μg for the second, third, and fourth injections). Mice in groups G-3 and G-4 were also injected intraperitoneally with 0.2 μg factor VIII on days 2-12. Blood samples for the antifactor VIII assay were obtained on days 20, 37, 58, and 82. Control groups G-1 and G-3 (○) were injected with only factor VIII. Groups G-2 and G-4 (•) were also injected intraperitoneally with 250 μg mCTLA4-Ig on the day before and the day after the first factor VIII injection. The antifactor VIII antibody concentration was determined by ELISA. Antifactor VIII assay data points indicated as less than 0.16 μg/mL were similar to those for plasma samples obtained from unimmunized hemophilia A mice.
The effect of mCTLA4-Ig on antifactor VIII antibody formation.
Four groups of hemophilia A mice were injected with recombinant human factor VIII on days 0, 23, 44, and 66 (initially 1 μg intravenously and then 0.2 μg for the second, third, and fourth injections). Mice in groups G-3 and G-4 were also injected intraperitoneally with 0.2 μg factor VIII on days 2-12. Blood samples for the antifactor VIII assay were obtained on days 20, 37, 58, and 82. Control groups G-1 and G-3 (○) were injected with only factor VIII. Groups G-2 and G-4 (•) were also injected intraperitoneally with 250 μg mCTLA4-Ig on the day before and the day after the first factor VIII injection. The antifactor VIII antibody concentration was determined by ELISA. Antifactor VIII assay data points indicated as less than 0.16 μg/mL were similar to those for plasma samples obtained from unimmunized hemophilia A mice.
To investigate if the limited duration of unresponsiveness is a result of the short half-life of human factor VIII in these mice (4-5 hours in murine hemophilia A8), control and mCTLA4-Ig–treated mice (groups G-3 and G-4, Figure 3) were injected with 1 μg factor VIII intravenously on day 0. This was followed by daily intraperitoneal injections of 1 μg factor VIII on days 2-12. On day 20, high-titer antifactor VIII was present in the control mice (group G-3), as determined by ELISA, and the mean inhibitor titer was 694 BU. In contrast, the group G-4 mice that were injected with mCTLA4-Ig on the day before and the day after the first exposure to factor VIII had no detectable antifactor VIII on day 20. The delayed antifactor VIII antibody response after 3 additional factor VIII injections was the same in these mice as that in the group G-2 animals. Thus, the limited persistence of factor VIII in the plasma after CTLA4-Ig injection was not the reason for a limited duration of unresponsiveness in CTLA4-Ig– treated mice.
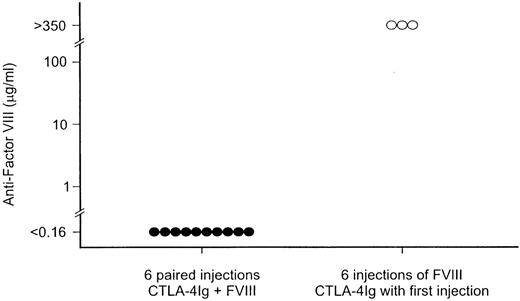
Because a delayed antifactor VIII response was detected after repeated factor VIII infusions when mCTLA4-Ig was given only at the time of the first factor VIII exposure, we determined if mCTLA4-Ig might prevent antifactor VIII development if given with each factor VIII infusion. In that experiment (Figure 4), hemophilia A mice were simultaneously infused 6 times at 3-week intervals with both factor VIII and mCTLA4-Ig. There was no detectable antifactor VIII in any of 10 mice treated in this manner when they were tested 4 weeks after the sixth factor VIII injection. In contrast, high-titer antifactor VIII was present in serum from mice that had received only 1 mCTLA4-Ig injection (at the time of the first exposure to factor VIII) followed by 5 injections of factor VIII alone.
The effect of repeated administration of mCTLA4-Ig on antifactor VIII antibody formation.
After the first injection, which contained both factor VIII and mCTLA4-Ig, hemophilia A mice were injected intravenously, at 3-week intervals, with both 1 μg factor VIII and 250 μg mCTLA4-Ig (•) or with factor VIII alone (○). Serum samples for the antifactor VIII assay were obtained 4 weeks after the sixth factor VIII injection.
The effect of repeated administration of mCTLA4-Ig on antifactor VIII antibody formation.
After the first injection, which contained both factor VIII and mCTLA4-Ig, hemophilia A mice were injected intravenously, at 3-week intervals, with both 1 μg factor VIII and 250 μg mCTLA4-Ig (•) or with factor VIII alone (○). Serum samples for the antifactor VIII assay were obtained 4 weeks after the sixth factor VIII injection.
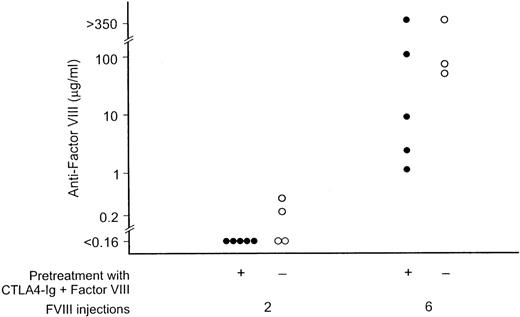
These mCTLA4-Ig–treated mice were then tested to determine if they would have an immune response after additional factor VIII injections in the absence of mCTLA4-Ig. After 2 intravenous injections at 3-week intervals, none of the 5 mice developed antifactor VIII, while low-level antifactor VIII was detected in 2 of 4 control mice not previously exposed to either factor VIII or mCTLA4-Ig (Figure5). After 6 injections of factor VIII alone, the mean factor VIII titer was 93 μg/mL for mice that had previously received both factor VIII and mCTLA4-Ig, while the mean titer was 155 μg/mL for the control mice. These data document the limits of antigen-specific immune suppression that followed from repeated coadministration of mCTLA4-Ig with factor VIII.
The effect of simultaneous administration of mCTLA4-Ig and factor VIII.
Hemophilia A mice treated as described for Figure 4, with 6 injections of both factor VIII and mCTLA4-Ig, were subsequently given 6 intravenous injections of 0.2 μg of factor VIII at 3-week intervals without additional mCTLA4-Ig (•). Control mice with no prior factor VIII exposure were immunized in parallel (○). Serum samples for the antifactor VIII assay were obtained 3 weeks after the second and sixth injections.
The effect of simultaneous administration of mCTLA4-Ig and factor VIII.
Hemophilia A mice treated as described for Figure 4, with 6 injections of both factor VIII and mCTLA4-Ig, were subsequently given 6 intravenous injections of 0.2 μg of factor VIII at 3-week intervals without additional mCTLA4-Ig (•). Control mice with no prior factor VIII exposure were immunized in parallel (○). Serum samples for the antifactor VIII assay were obtained 3 weeks after the second and sixth injections.
Secondary immune response to factor VIII suppressed by mCTLA4-Ig
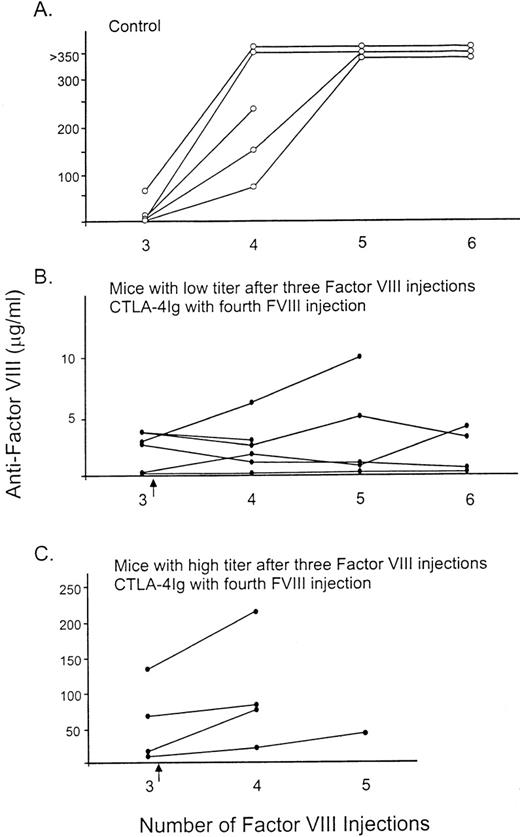
To determine if mCTLA4-Ig modifies a secondary immune response to factor VIII, we injected mCTLA4-Ig at the same time that factor VIII was given to hemophilia A mice that had already developed antifactor VIII. Initially, all the hemophilia A mice had been injected 3 times with 0.2 μg factor VIII, and the level of antifactor VIII was determined by ELISA. The control mice then received 3 additional injections of factor VIII, while the remaining mice were given mCTLA4-Ig at the same time as they received the first of 3 additional factor VIII injections. While many mice died of bleeding complications during this experiment because of the repeated injections and blood sample collections, the results were clearly different for the 2 groups. An increase in the antifactor VIII titer was noted after the fourth injection of factor VIII for the control mice, with the mean titer ranging from 16 to 230 μg/mL (Figure6A). After the fifth factor VIII injection, all antifactor VIII titers were greater than 350 μg/mL in the 4 remaining control mice. In contrast, mice treated with mCTLA4-Ig at the fourth factor VIII injection had minimal or no increases in antifactor VIII (Figure 6B and C). The administration of mCTLA4-Ig (Figure 6C) inhibited this secondary immune response to factor VIII in mice that had already developed relatively high antifactor VIII levels (5-90 BU). This also occurred in mice with minimal antifactor VIII after the 3 initial injections (less than 5 BU; Figure 6B).
The effect of mCTLA4-Ig on the secondary immune response to factor VIII.
All mice initially received 3 intravenous injections of 0.2 μg factor VIII at 2-week intervals. (A) Control mice (○) were then injected with factor VIII an additional 3 times, and blood samples were obtained for assay. (B, C) The other mice (•) were injected intraperitoneally with 250 μg mCTLA4-Ig the day before and the day after the fourth factor VIII injection (as indicated by the arrow). This was followed by 2 more injections of only factor VIII at 3-week intervals. The number of factor VIII injections prior to the blood sample tested for antifactor VIII is indicated on the horizontal axis.
The effect of mCTLA4-Ig on the secondary immune response to factor VIII.
All mice initially received 3 intravenous injections of 0.2 μg factor VIII at 2-week intervals. (A) Control mice (○) were then injected with factor VIII an additional 3 times, and blood samples were obtained for assay. (B, C) The other mice (•) were injected intraperitoneally with 250 μg mCTLA4-Ig the day before and the day after the fourth factor VIII injection (as indicated by the arrow). This was followed by 2 more injections of only factor VIII at 3-week intervals. The number of factor VIII injections prior to the blood sample tested for antifactor VIII is indicated on the horizontal axis.
Discussion
We used a mouse model of hemophilia A to establish the T-cell dependence of inhibitory antibody formation to factor VIII and to evaluate the potential use of a reagent that blocks the B7/CD28/CTLA4 costimulation pathway. The murine fusion protein, mCTLA4-Ig, blocks this pathway, and it prevented the initiation of the antibody response and suppressed the development of a secondary antibody response to factor VIII. These data gave direct evidence, for the first time, that the antibody response to factor VIII is T-cell dependent and can be modulated by costimulatory blockade.
While these studies in mice were done with recombinant human factor VIII, we believe that they are relevant for our efforts to prevent and treat factor VIII inhibitor antibodies in patients with hemophilia A. As a practical matter, mouse factor VIII is not available in the quantity and purity needed to carry out these experiments. While the gene has been cloned, the protein has not been produced in quantity by recombinant technology. Moreover, as the hemophilic mice lack mouse factor VIII, having no detectable factor VIII activity in their plasma and no detectable factor VIII light chain protein by immunoassay,17 they would be expected to respond to both human and mouse factor VIII as completely foreign proteins. While it is possible that there are some “mouse-specific” determinants on murine factor VIII that are shared with other mouse proteins, this would only make the response to human factor VIII somewhat greater than that to mouse factor VIII. However, since we have demonstrated that the blockade of a costimulatory pathway can prevent immunization, the response to human factor VIII would be, if anything, more difficult to prevent.
To the extent possible, the experimental design incorporated all concerns that may be important in the application of costimulatory blockade in patients. For this reason, the dose chosen for most studies, 0.2 μg factor VIII per intravenous injection, was comparable to that of a single therapeutic dose given to a patient with severe hemophilia A (with the goal of raising the plasma factor VIII level to 100%). For the initial experiments, the blockade was limited to a single pair of injections of mCTLA4-Ig at the time of the first factor VIII exposure, an approach that would be expected to have less effect on the patient's overall immunologic status than repeated CTLA4-Ig administration at each factor VIII injection. However, our experiments showed that an immune response to factor VIII returned 6-9 weeks after a single pair of CTLA4-Ig injections (Figure 1) or even after a series of 6 paired injections of factor VIII and CTLA4-Ig at 3-week intervals (Figure 3). The latter experiment did demonstrate, however, that blockade of antifactor VIII antibody was maintained while CTLA4-Ig was given at 3-week intervals over a 5- month period (Figure 2).
It is not certain why the hemophilic mice formed antifactor VIII after they were given additional factor VIII injections in the absence of mCTLA4-Ig. However, similar reversal of specific unresponsiveness to a T-dependent antigen (sheep red blood cells [SRBC]) has been described by Wallace and colleagues.24 In those studies, a single dose of murine CTLA4-Ig prevented antibody formation immediately after the first and second SRBC injections, but a high-titer response followed the third SRBC injection. Their experiments also showed that unresponsiveness to SRBC persisted while mice were successfully immunized with a different T-dependent antigen. Finally, T and B cells from unresponsive mice were shown to be functional when transferred to irradiated mice subsequently immunized with SRBC. Thus, they had been neither depleted of responsive cells nor rendered permanently “tolerant” by CTLA4-Ig.24
In our studies, the development of antifactor VIII after repeated antigen exposure is likely to be due to the combination of mCTLA4-Ig clearance over time (its serum half-life β-phase being 6 days24) and emigration from the thymus of naive T cells that are capable of an antifactor VIII response.25 However, Wallace et al24 found that mice thymectomized before the initial exposure to SRBC and CTLA4-Ig also develop anti-SRBC after the third injection. The down-regulatory role of B7-CTLA4 interactions is also important in the induction and maintenance of T-cell anergy.14 Thus, CTLA4-Ig blockade of the B7-CTLA4 interaction could also prevent maintenance of anergy in hemophilia A mice.
Blockade of CD28/B7 interactions using either CTLA4-Ig or anti-B7 antibodies has previously been shown to be an effective method for blocking T-cell responses in several in vivo animal models of transplantation and autoimmunity9 and in recent clinical studies.15,16,26 27 CD28 is expressed constitutively on T cells, and the ligands for CD28, B7-1 and B7-2, are expressed on B cells as well as other antigen-presenting cells such as dendritic cells and monocytes. Blockade of CD28 signaling in T cells results in a failure in the activation of the antigen-specific T-cell subset.
In the absence of CD28 costimulation and IL-2 production, T-cell activation does not occur efficiently. In vitro, exposure of T cells to antigens in the absence of costimulation can render a T cell unresponsive to reactivation, a condition termed anergy.28Whether this mechanism is effective in vivo is unknown. Additional effects of costimulation blockade on the T-cell–B-cell interactions in the germinal centers may be critical in preventing antibody formation.
Because the blockade of the B7-1 and B7-2 ligands individually can lead to distinct effects in models of autoimmune disease, it has been suggested that B7-1 and B7-2 may have unique biological roles.29 30 Alternatively, the earlier expression of B7-2 has suggested a greater role for B7-2 in initiating a T-cell response and a role for B7-1 in amplifying or regulating a T-cell response. We have examined the roles of B7-1 and B7-2 in the initiation of the antifactor VIII antibody response using hemophilia A mice deficient for the expression of either B7-1 or B7-2. Although the antibody response was not significantly altered in the B7-1 deficient mice, the B7-2 deficient mice formed no detectable antifactor VIII antibodies (Figure1). In addition, the T-cell proliferative response to factor VIII was greatly suppressed in the B7-2 deficient mice, although it was normal in B7-1 deficient mice (Figure 2). This indicates that the B7-2 ligand is critical for T-cell priming in hemophilia A mice given factor VIII intravenously.
B7-2 is expressed in lymph node germinal centers of both humans and mice,31,32 and the blockade of B7-2 in mice has been shown to block antibody affinity maturation in germinal centers and to impair the development of B-cell memory.32 Moreover, studies of the T-cell–dependent antibody response to the trinitrophenol (TNP) hapten in B7-1– and B7-2–deficient mice indicated that B7-2 is essential for isotype switching and for the formation of germinal centers when antigen is given intravenously and in the absence of an adjuvant.18 Under those conditions of antigen administration, B7-1 did not compensate for the absence of B7-2, while B7-1–deficient mice generated an antibody response comparable to wild type mice.14,18 However, mice lacking either B7-1 or B7-2 antigens had high-titer TNP-specific IgG responses when immunized subcutaneously with TNP in complete Freund's adjuvant. Mice lacking both B7-1 and B7-2 antigens, however, failed to class switch or form germinal centers when immunized using these conditions. Thus, B7-1 and B7-2 antigens can have compensating, overlapping roles for isotype switching and germinal center formation when inflammation leads to up-regulation of B7-1 and B7-2 expression.14 18
The formation of inhibitory antibodies to factor VIII is a critical problem for hemophilia A patients treated with protein replacement therapy and may be a major problem for gene therapy in hemophilic patients. The results of our studies with the hemophilia A mouse suggest that a costimulation blockade may be an effective therapy for the prevention of antifactor VIII antibodies in these patients. Data from the B7-2–deficient mice suggest that the blockade of the CD28–B7-2 interaction is more important in the prevention of an antibody response to factor VIII injected intravenously and that anti–B7-2 may be as effective as CTLA4-Ig in blocking the initiation of an antifactor VIII response. If this is the case, B7-1 function would remain intact, and the treatment could be less generally immunosuppressive than would be the case if CTAL4-Ig were used.
Although we have not verified that the antibody-free hemophilia A mice treated with CTLA4-Ig have normal factor VIII recovery after an infusion of factor VIII, published studies have addressed this question.33 Using immunologic and coagulation-based assays, gene therapy experiments with this mouse model of hemophilia A have documented normal factor VIII recovery and clearance in mice treated with a human factor VIII–encoding adenoviral vector when their plasma was free of antifactor VIII.33
At the present time, it is not possible to identify which hemophilia A patients will develop inhibitory antibodies after factor VIII treatment.1,2 For this reason, the clinical application of our studies would be primarily for patients who have already developed detectable antifactor VIII. In evaluating this possibility, the ability of anti–B7-2 to block antibody-affinity maturation32suggests that blockade of the B7/CD28 pathway might be effective in suppressing a secondary antifactor VIII antibody response, at least in part, by preventing maturation of the B-cell response. In the single experiment reported here (Figure 6), combined treatment of hemophilia A mice with CTLA4-Ig and factor VIII after detection of antifactor VIII antibody prevented a further increase in antibody titer in most mice and a fall in titer in some. If the pattern is similar in patients, B7-2 costimulation blockade at the time of factor VIII treatment may stabilize the inhibitor level for patients treated early after detection of antifactor VIII. For low-titer factor VIII inhibitor patients, this costimulation blockade might then permit an escalation of factor VIII doses to therapeutically effective levels without further boosting the inhibitor titer. As antibody suppression by costimulation blockade appears to last several weeks, intermittent dosing may then be sufficient to prevent or modulate inhibitor formation. In this way, therapeutic costimulation blockade would reduce antifactor VIII while leaving the immune responses to other antigens intact.
Acknowledgments
We thank G. Gray for helpful discussions, Baxter Healthcare for providing recombinant human factor VIII, Marina Borovok for technical assistance, and Carolyn Murray and Debbie Wilder for manuscript preparation.
Supported in part by grants HL 36099 (L.W.H.) and AI 38310 (A.H.S.) from the National Institutes of Health, Bethesda, MD.
Reprints:Leon W. Hoyer, Holland Laboratory, American Red Cross, 15601 Crabbs Branch Way, Rockville, MD 20855; email:hoyer@usa.redcross.org.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 U.S.C. section 1734.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal