To the editor:
Our previous study showed HIV envelope glycoprotein induces killing of CD4-negative Vγ2Vδ2 (referred as Vδ2) T cells by binding to CCR5 and α4β7.1 Blocking either CCR5 with receptor antagonists or α4β7 with MAdCAM1 reduced Env binding and killing of Vδ2 cells. Signaling through p38 and caspase activation were responsible for envelope-dependent cell death. However, we could not determine which of these receptors mediated death signaling. We have now investigated effects of HIV envelope on the CD4-negative Vδ1 subset of γδ T cells, which express α4β7, but not CCR5 receptor. Comparing envelope receptors and responses on Vδ1 and Vδ2, cells showed the envelope-α4β7 interaction does not generate a death signal.
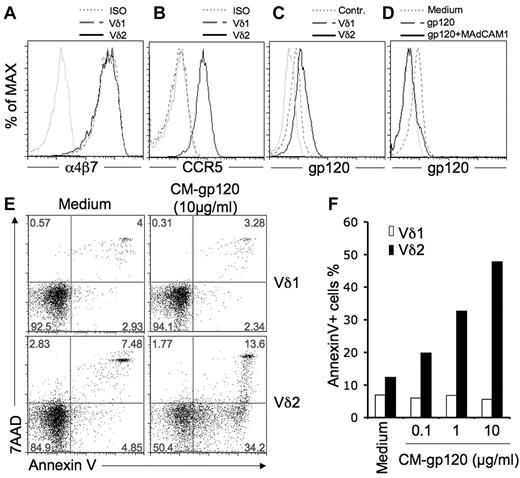
Vδ1 and Vδ2 are 2 major types of human γδ T cells. For healthy adults, the ratio of Vδ2:Vδ1 cells in blood is 3:10.2 The Vδ2:Vδ1 cell ratios are inverted among HIV-infected individuals because Vδ2 cells are depleted and the Vδ1 subset is expanded.2-4 Both Vδ1 and Vδ2 are CD4-negative and nonpermissive for HIV infection.2 We proposed that HIV envelope–induced T-cell death might be an important mechanism for Vδ2-cell depletion in HIV disease,1 but we do not know the effects on Vδ1 cells that are increased during HIV disease. First, we tested for HIV-receptor expression on Vδ1 T cells. Vδ1 cells did not express dendritic cell–specific intercellular adhesion molecule-3-grabbing nonintegrin (DC-SIGN) or mannose receptor (data not shown), but had levels of α4β7 similar to those found on Vδ2 cells (Figure 1A),1 CCR5, which is highly expressed on Vδ2 cells,1 was not detected on Vδ1 cells (Figure 1B). HIV envelope was bound to Vδ1 cells, but at lower levels compared with Vδ2 cells (Figure 1C). Binding was blocked completely by MAdCAM1 (Figure 1D). Next, we tested whether 3 different R5-tropic HIV envelope glycoproteins (from BaL, CN54, and CM strains) induced killing of Vδ1 cells. We reported that BaL and CN54 glycoproteins induced cell death among Vδ2 T cells.1 Here we show that CM-gp120 treatment killed 50% of Vδ2 T cells based on 7-aminoactinomycin-D and annexin V staining (Figure 1E-F), but had no effect on Vδ1 T cells (Figure 1E-F).
HIV envelope binding to α4β7 does not generate a death signal in γδ T cells. (A) Vδ1 or Vδ2 T cells express similar levels of a α4β7 integrin. Cells were stained with primary mouse anti–human α4β7 antibody (NIH AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: α4β7 monoclonal antibody from Dr A. A. Ansari; clone Act-1), then washed and stained with a secondary APC-conjugated rat anti–mouse antibody (BD Biosciences; clone X56). (B) Vδ1 T cells do not express CCR5. Cells were stained with PE-conjugated antibodies for CCR5 (BD Biosciences; clone 2d7) and isotype control, then analyzed by flow cytometry. (C) Vδ1 and Vδ2 T cells bind HIV gp120. Cells were stained with the fluorescein-conjugated HIV gp120 (YU2; Immno Diagnostics) in medium. (D) gp120 binding to Vδ1 T cells was blocked by the α4β7 ligand MAdCAM1. Cells were incubated with or without MAdCAM1 (20 μg/mL; R&D Systems), for 1 hour before staining with fluorescein-conjugated HIV gp120 (YU2). (E) CM-gp120 induces annexin V expression on Vδ2 but not Vδ1 cells. Cells were incubated with or without soluble HIV gp120 glycoprotein (CM strain; 10 μg/mL; NIH AIDS Research and Reference Reagent Program; and HIV-1 gp120 CM envelope protein from Protein Sciences Corp) for 24 hours, then stained with annexin V (BD Biosciences) and 7-aminoactinomycin-D (BD Biosciences). Data are representative of 3 independent experiments. (F) CM-gp120 induced annexin V expression and cell death only in CCR5+ Vδ2 cells.
HIV envelope binding to α4β7 does not generate a death signal in γδ T cells. (A) Vδ1 or Vδ2 T cells express similar levels of a α4β7 integrin. Cells were stained with primary mouse anti–human α4β7 antibody (NIH AIDS Research and Reference Reagent Program, Division of AIDS, NIAID, NIH: α4β7 monoclonal antibody from Dr A. A. Ansari; clone Act-1), then washed and stained with a secondary APC-conjugated rat anti–mouse antibody (BD Biosciences; clone X56). (B) Vδ1 T cells do not express CCR5. Cells were stained with PE-conjugated antibodies for CCR5 (BD Biosciences; clone 2d7) and isotype control, then analyzed by flow cytometry. (C) Vδ1 and Vδ2 T cells bind HIV gp120. Cells were stained with the fluorescein-conjugated HIV gp120 (YU2; Immno Diagnostics) in medium. (D) gp120 binding to Vδ1 T cells was blocked by the α4β7 ligand MAdCAM1. Cells were incubated with or without MAdCAM1 (20 μg/mL; R&D Systems), for 1 hour before staining with fluorescein-conjugated HIV gp120 (YU2). (E) CM-gp120 induces annexin V expression on Vδ2 but not Vδ1 cells. Cells were incubated with or without soluble HIV gp120 glycoprotein (CM strain; 10 μg/mL; NIH AIDS Research and Reference Reagent Program; and HIV-1 gp120 CM envelope protein from Protein Sciences Corp) for 24 hours, then stained with annexin V (BD Biosciences) and 7-aminoactinomycin-D (BD Biosciences). Data are representative of 3 independent experiments. (F) CM-gp120 induced annexin V expression and cell death only in CCR5+ Vδ2 cells.
The α4β7 integrin binds gp120 but does not mediate HIV envelope–induced death signaling. HIV envelope did not activate p38 or caspase in Vδ1 T cells (data not shown), showing that α4β7 does not signal the death pathway in the absence of CCR5. In Vδ2 cells, α4β7 may enhance killing by increasing the avidity of gp120 binding, but CCR5 is the key signaling receptor for cell death. These findings explain the differential effects of gp120 on CD4-negative Vδ2 versus CD4-negative Vδ1 T cells, which are mirrored in HIV patients where only Vδ2 T cells are depleted.
Authorship
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: C. David Pauza, PhD, Institute of Human Virology, University of Maryland School of Medicine, 725 W Lombard St, N546, Baltimore, MD, 21201; e-mail: cdpauza@ihv.umaryland.edu.
References
National Institutes of Health

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal