Abstract
This multicenter, first-in-human study evaluated the safety, tolerability, and pharmacokinetic and pharmacodynamic properties of the anti-CS1 monoclonal antibody elotuzumab. A standard 3 + 3 design was used to determine maximum tolerated dose; dose-limiting toxicities were assessed during cycle 1. Thirty-five patients with relapsed/refractory multiple myeloma were treated with intravenous elotuzumab at doses ranging from 0.5 to 20 mg/kg every 2 weeks. Patients who achieved at least stable disease after 4 treatments could receive another 4 treatments. No maximum tolerated dose was identified up to the maximum planned dose of 20 mg/kg. The most common adverse events, regardless of attribution, were cough, headache, back pain, fever, and chills. Adverse events were generally mild to moderate in severity, and adverse events attributed to study medication were primarily infusion-related. Plasma elotuzumab levels and terminal half-life increased with dose whereas clearance decreased, suggesting target-mediated clearance. CS1 on bone marrow–derived plasma cells was reliably saturated (≥ 95%) at the 10-mg/kg and 20-mg/kg dose levels. Using the European Group for Bone and Marrow Transplantation myeloma response criteria, 9 patients (26.5%) had stable disease. In summary, elotuzumab was generally well tolerated in this population, justifying further exploration of this agent in combination regimens.
Introduction
Multiple myeloma (MM) is an incurable malignancy arising from postgerminal mature B cells, characterized by an excess of monotypic plasma cells in the bone marrow and elevated levels of monoclonal immunoglobulins in the serum and/or urine.1 Common clinical sequelae include lytic bone lesions, fractures, myelosuppression, and renal failure. In the United States, the estimated annual diagnosed incidence is 20 000, with a prevalence of approximately 62 000 as of 2007.2,3 MM accounts for 15% of all hematologic malignancies and 2% of all malignancies.4 Advances in high-dose chemotherapy and stem cell transplantation have improved overall survival (OS) and event-free disease periods in MM,5-7 although relapses are inevitable. Newer therapeutic agents, such as bortezomib, thalidomide, and lenalidomide, have demonstrated clinical benefit in patients with newly diagnosed8-13 and relapsed or refractory disease.5,14,15 Despite these therapeutic advances, long-term control of relapsed/refractory MM remains elusive for most patients. Progressive disease that is resistant to both immunomodulatory drugs and bortezomib is associated with a particularly poor prognosis.16 As such, there remains an important need for additional novel therapies to augment existing first-generation agents and continue to improve patient outcome.
Elotuzumab is a humanized monoclonal IgG1 antibody directed against human CS1 (also known as CD2 subset-1, SLAMF7, CRACC, and CD319), a cell surface antigen glycoprotein that is highly expressed on MM cells and normal plasma cells. CS1 is expressed at a lower level on natural killer (NK) cells, NK-like T (NKT) cells, and a subset of CD8 positive T cells. Little to no expression is detected on resting B cells, monocytes, CD4+ T cells, granulocytes, hematopoietic stem cells, and epithelia, vessels, or smooth muscle cells of all tissues.17
Elotuzumab has significant in vivo antitumor efficacy in severe-combined immunodeficient (SCID) mice MM xenograft models.17-19 Data obtained from these models and in vitro studies using human peripheral blood mononuclear cells formed the basis for the selection of the doses and schedule used in the current study.
This study was conducted to determine the maximum tolerated dose of elotuzumab up to a maximum planned dose (MPD) of 20 mg/kg when given as a monotherapy and to characterize the safety, pharmacokinetics (PK), and preliminary efficacy in adults with advanced MM and limited treatment options.
Methods
Study design
This was a multicenter, open-label, dose-escalation phase 1 study designed to evaluate elotuzumab administered by intravenous infusion at up to 6 dose levels (0.5, 1.0, 2.5, 5.0, 10, or 20 mg/kg) in patients with advanced MM. Patients received elotuzumab once every 14 days for 8 weeks. Patients who did not show evidence of progressive disease (PD) or relapse at week 8 had the option of receiving a second 8-week treatment course at the same dose level and schedule. After completion of treatment, patients were evaluated for adverse events (AEs) at 30 days and 60 days after last elotuzumab dose. The study used a 3 + 3 dose escalation approach with each cohort starting with 3 patients. For the first cohort, there was a 14-day observation period between the first doses administered to each of the initial 3 patients enrolled. Patients in subsequent dose cohorts could be dosed simultaneously. If no dose-limiting toxicity (DLT) was observed within the initial 28-day dosing period, enrollment commenced at the next higher dose level, to an MPD of 20 mg/kg. If 1 patient in the cohort had a DLT, 3 additional patients were enrolled in the cohort. If no additional DLT occurred, patient enrollment to the next dose level proceeded. If a second patient in a cohort had a DLT, study treatment in that cohort was to be stopped. An independent Data Safety Monitoring Board reviewed the safety data for each cohort before patients being enrolled into the next cohort. DLT was defined as any grade 3 or greater elotuzumab-related AE (National Cancer Institute Common Terminology Criteria for Adverse Events Version 3) occurring during the first 28 days of therapy.
Elotuzumab was prepared according to protocol specifications and infused at a rate of 2 mL/min with calibrated infusion pumps. Originally, pretreatment with an antihistamine and acetaminophen was optional in patients who experienced any infusion reaction. After the observation of infusion-related AEs, the protocol was amended to require that all patients receive an antihistamine and acetaminophen before and during each infusion. In addition, unless contraindicated, an intravenous corticosteroid was required to be administered before the first dose in 20 mg/kg elotuzumab cohort.
The study was conducted in compliance with principles of the International Conference on Harmonisation guidelines on Good Clinical Practice, the Declaration of Helsinki, and the applicable local and national regulations. All patients provided written informed consent.
The sponsor conducted the statistical analysis. Laboratory tests were conducted at 3 central laboratories (CRL Medinet Inc, Mayo Laboratories, and Esoterix Inc). In addition, PK and immunogenicity tests were conducted by the sponsor.
Patients were monitored for safety by assessing all AEs according to the National Cancer Institute Common Terminology Criteria for Adverse Events Version 3.0 until 60 days after the last dose of elotuzumab. Other safety evaluations included complete blood count and serum chemistries, vital signs, laboratory tests, chest x-ray, electrocardiogram, physical examinations, Eastern Cooperative Oncology Group (ECOG) performance status assessment, urinalysis, prothrombin time/partial prothromboplastin time, 2-dimensional echocardiogram, skeletal survey, and bone marrow aspirate or biopsy. Safety data were monitored on an ongoing basis by an independent Data Safety Monitoring Board.
Treatment responses were determined by the investigator using the European Group for Blood and Marrow Transplantation (EBMT) myeloma response criteria,20 which included measurements of MM plasma cell levels in the bone marrow, serum and urine M-protein, and serum and urinary free light chain. Patients were evaluated for disease activity and survival up to 6 months after their last dose.
PK
Blood samples were collected throughout the study and until 60 days after dose for the determination of PK parameters. Serum concentrations of elotuzumab were assessed by an ELISA (assay range, 75-2000 ng/mL). PK parameters, such as maximum serum concentration (Cmax), terminal phase half-life (T1/2λ), systemic clearance (CL), area under the concentration-time curve (AUC) from time zero to infinity (AUCinf), and AUC for the dosing interval (AUCτ), were estimated using noncompartmental methods in Phoenix WinNonlin Version 6.0 (Pharsight Corporation) using the constant intravascular infusion model (model 202).
Immunogenicity
Immunogenicity testing was performed on samples collected up to 60 days after treatment using a tiered approach. All samples were initially screened for the presence of antibodies to elotuzumab (antidrug antibody [ADA]) using an electrochemiluminescent bridging assay. The screening assay cut-point21 was set using baseline samples from patients in the study and additional drug-naive MM serum. Screen-positive samples were titered and then tested in a confirmatory assay by preincubating with soluble, excess elotuzumab to determine whether a significant decrease in signal was observed. Confirmed positive samples were then tested in a cell-based neutralizing antibody (NAb) assay. The NAb assay format uses ruthenium labeled elotuzumab that binds CS1 on cells in the presence of patient's baseline serum; if NAbs are present at later time points, a percentage inhibition in signal relative to the baseline response can be observed. A sample was considered NAb-positive when the relative percentage inhibition was greater than or equal to 17.41% (cut-off based on the variation observed in the assay during validation).
Pharmacodynamics
CS1 saturation assays and lymphocyte counts.
Whole blood and bone marrow samples collected from patients at protocol-defined time points were shipped by overnight courier to Esoterix Clinical Trial Services. Percentage and absolute numbers of peripheral blood T, B, and NK cells were analyzed using the BD TruCOUNT assay (BD Biosciences). CS1 saturation was measured using fluorescently labeled elotuzumab in a flow cytometric assay. CS1 expression was assessed using a fluorescently labeled monoclonal antibody that does not compete with elotuzumab for binding to CS1. CS1 saturation and expression assays were performed on peripheral NK and CD8+ T cells and on bone marrow NK cells, as well as CD38+ and CD138+ putative myeloma cells.
Cytokines.
Serum levels of interferon-inducible protein 10 (IP-10), a chemokine that stimulates migration of activated T cells and NK cells, were measured in a multiplexed assay using LINCO kits on the Luminex platform (Linco Research). Samples were collected on the same day before elotuzumab treatment and at 2 and 4 hours after elotuzumab dosing, and then frozen until the assay was performed.
Statistical methods
The primary objective of the study was to evaluate the safety and identify the maximum tolerated dose of elotuzumab, to an MPD of 20 mg/kg, in patients with advanced MM. Secondary objectives were to evaluate the PK, immunogenicity, and objective response rates determined by the EBMT criteria. Exploratory objectives included: evaluations of CS1 expression on bone marrow MM cells and peripheral NK, NKT, and CD8+ T cells; and evaluation of biomarkers, including prognostic factors, such as serum β2-microglobulin (β2M) and serum albumin.
The modified intent-to-treat population was defined as patients who have received at least 1 dose of study medication. A patient was considered DLT evaluable if the patient had received 2 doses of study medication or had experienced a DLT resulting from the first treatment.
Patient eligibility
Patients eligible for the study included adults 18 years of age or older who had a diagnosis of advanced MM and prior treatment with at least 2 prior MM therapies. Patients were also required to meet the following criteria: ECOG performance status score of 0 to 2; measurable M component in serum (≥ 0.5 g/dL) and/or urine (≥ 0.2 g excreted in a 24-hour collection sample); adequate bone marrow function (defined as absolute neutrophil count > 1000 cells/mm3, platelets ≥ 75 000 cells/mm3, and hemoglobin ≥ 8 g/dL); serum calcium (corrected for albumin) level within normal range; left ventricular ejection fraction more than or equal to 45% (2-dimensional echocardiogram or multiple-gated acquisition scan within 30 days before first dose of study drug); negative pregnancy test within 48 hours before first dose in women of childbearing potential; serum alanine aminotransferase or aspartate aminotransferase ≤ 3× the upper limit of normal, total bilirubin ≤ 2× the upper limit of normal (unless related to MM); and serum creatinine ≤ 2.0 mg/dL (unless related to MM, then ≤ 3.0 mg/dL).
Patients were ineligible for this study if they had met any 1 of the following criteria: life expectancy of less than 3 months; prior malignancy (except for adequately treated basal cell or squamous cell skin cancer, in situ cervical cancer, or other cancer from which the patient had been disease-free for at least 5 years); plasma cell leukemia (active or prior); uncontrolled medical problems, such as diabetes mellitus, coronary artery disease, hypertension, unstable angina, arrhythmias, and pulmonary, hepatic, and renal diseases, unless renal insufficiency was thought to be secondary to MM; solitary bone or solitary extramedullary plasmacytoma as the only evidence of plasma cell dyscrasia; corticosteroid, bortezomib, or other proteasome inhibitor, thalidomide, lenalidomide, or melphalan within 2 weeks of the first dose of elotuzumab; nitrogen mustard agents within 6 weeks of the first dose of elotuzumab; investigational drug within 4 weeks or 5 half-lives (whichever was greater) of the first dose of elotuzumab; stem cell or bone marrow transplant within 12 weeks before the first dose of elotuzumab; biologic agents, including intravenous immunoglobulin and monoclonal antibodies within 4 weeks of the first dose of elotuzumab; neuropathy more than grade 2; symptomatic orthostatic hypotension; evidence of amyloidosis; known active infections requiring antibiotics, antivirals, or antifungals; serious psychiatric illness, active alcoholism, or drug addiction that may hinder or confuse follow-up evaluation; hypersensitivity to recombinant proteins or excipients in the investigational agent; platelet transfusion within 72 hours of obtaining screening platelet count; and any condition that in the investigator's opinion made the patient unsuitable for study participation.
To mitigate infusion reactions, the original protocol was amended to include a premedication regimen; patients in the 20-mg/kg elotuzumab cohort received premedication of 50 mg methylprednisolone, diphenhydramine, and acetaminophen before the first elotuzumab dose only. Diphenhydramine and acetaminophen were suggested before each subsequent infusion. Another amendment allowed patients who demonstrated stable disease or better at day 56 to receive additional doses of elotuzumab.
Results
Study population
The study was conducted between November 2006 and August 2009 at 8 sites in the United States and 1 in France. Study disposition is detailed in Table 1. Fifty-one patients were screened, of which 35 were enrolled into 6 dosing cohorts: 3 in the 0.5-mg/kg cohort, 4 in the 1.0-mg/kg cohort, 6 in the 2.5-mg/kg cohort, 4 in the 5.0-mg/kg cohort, 4 in the 10-mg/kg cohort, and 14 in the 20-mg/kg cohort. Thirty-four patients received study treatment. One patient was enrolled in the 10-mg/kg cohort but was withdrawn from the study by the investigator before receiving study medication after developing severe neuropathy. One patient each in the 1.0-mg/kg and 5.0-mg/kg cohorts withdrew from the study because of disease progression after receiving one or 2 doses. In each of these 3 cohorts, another patient was enrolled as a replacement.
Patient disposition (ITT population: total N = 35)
| . | No. (%) . |
|---|---|
| Received study drug | 34 (97.1) |
| Completed initial treatment phase | 25 (71.4) |
| Received re-treatment | 8 (22.9) |
| Completed follow-up period | 16 (45.7) |
| Completed treatment | 6 (17) |
| Reasons for discontinuation | |
| AE | 1 (2.9) |
| Disease progression | 20 (57.1) |
| DLT | 2 (5.7) |
| Investigator's decision | 2 (5.7) |
| Patient's decision | 2 (5.7) |
| Other | 2 (5.7) |
| . | No. (%) . |
|---|---|
| Received study drug | 34 (97.1) |
| Completed initial treatment phase | 25 (71.4) |
| Received re-treatment | 8 (22.9) |
| Completed follow-up period | 16 (45.7) |
| Completed treatment | 6 (17) |
| Reasons for discontinuation | |
| AE | 1 (2.9) |
| Disease progression | 20 (57.1) |
| DLT | 2 (5.7) |
| Investigator's decision | 2 (5.7) |
| Patient's decision | 2 (5.7) |
| Other | 2 (5.7) |
ITT indicates intent to treat.
Patient demographics and disease characteristics are presented in Table 2. Most patients were white, with a median age of 64.5 years and a median of 4.5 prior MM treatments. All patients had received prior immunomodulatory agents; 28 (82.4%) and 27 (79.4%) had received lenalidomide and thalidomide, respectively. Twenty-eight (82.4%) patients had received prior bortezomib.
Patient demographics and baseline disease characteristics (MITT population: total N = 34)
| Demographic/characteristic . | Value . |
|---|---|
| Median age, y (range) | 64.5 (46-87) |
| Race, n (%) | |
| Asian | 2 (5.9) |
| Black of African heritage | 3 (8.8) |
| White | 29 (85.3) |
| Sex, n (%) | |
| Male | 17 (50.0) |
| Female | 17 (50.0) |
| ECOG status, n (%) | |
| 0 | 14 (41.2) |
| 1 | 13 (38.2) |
| 2 | 6 (17.6) |
| 3 | 0 |
| 4 | 1 (2.9) |
| Median time since first diagnosis, y (range) | 4.4 (0.9-12.8) |
| Prior multiple myeloma treatments | |
| Median no., (range) | 4.5 (2-10) |
| Lenalidomide, n (%) | 28 (82.4) |
| Thalidomide, n (%) | 27 (79.4) |
| Bortezomib, n (%) | 28 (82.4) |
| Demographic/characteristic . | Value . |
|---|---|
| Median age, y (range) | 64.5 (46-87) |
| Race, n (%) | |
| Asian | 2 (5.9) |
| Black of African heritage | 3 (8.8) |
| White | 29 (85.3) |
| Sex, n (%) | |
| Male | 17 (50.0) |
| Female | 17 (50.0) |
| ECOG status, n (%) | |
| 0 | 14 (41.2) |
| 1 | 13 (38.2) |
| 2 | 6 (17.6) |
| 3 | 0 |
| 4 | 1 (2.9) |
| Median time since first diagnosis, y (range) | 4.4 (0.9-12.8) |
| Prior multiple myeloma treatments | |
| Median no., (range) | 4.5 (2-10) |
| Lenalidomide, n (%) | 28 (82.4) |
| Thalidomide, n (%) | 27 (79.4) |
| Bortezomib, n (%) | 28 (82.4) |
MITT indicates modified intent to treat.
Of the 34 patients treated, 25 completed the initial treatment phase and 8 were subsequently retreated. A median of 4 infusions were administered (range, 1-12). Reasons for treatment cessation are detailed in Table 1. The majority of patients discontinued treatment because of disease progression. In addition to the 2 patients who discontinued treatment because of DLTs (detailed in “AEs”), 1 patient in the 0.5-mg/kg cohort discontinued because of unrelated AEs of headache and congestive cardiac failure, which required hospitalization. Sixteen patients completed the 6-month posttreatment follow-up period.
Safety
Two patients experienced DLTs during their first cycle of treatment: 1 patient in the 2.5-mg/kg cohort had an episode of grade 3 increased serum creatinine, and 1 patient in the 20-mg/kg cohort experienced a grade 3 hypersensitivity reaction that resolved spontaneously within 24 hours. Subsequently, both patients discontinued study treatment. As a consequence, the 2.5-mg/kg cohort was expanded to 6 patients. A maximum tolerated dose was not reached in this study up to the MPD of 20 mg/kg.
AEs
Overall, 30 patients (88.2%) reported treatment-emergent AEs. The most frequent treatment-emergent AEs, regardless of attribution to study medication, included chills, fatigue, pyrexia, cough, headache, anemia, nausea, and back pain. Most events were grade 1 or 2 in severity. Eighteen patients (52.9%) experienced AEs attributed to elotuzumab. Table 3 details the most frequent treatment-related AEs. Thirty-one serious AEs were reported in 15 patients (44.1%). Six serious AEs occurring in 4 patients were assessed as related to treatment with elotuzumab: bradycardia (grade 2), chest discomfort (grade 2), chills (grade 2), hypersensitivity (grade 3), pyrexia (grade 2), and acute renal failure (grade 4), which was treated with medications, resolved to grade 3 at 5 days later, and required dialysis after the patient's discharge from the hospital.
Treatment-related AEs in more than 5% of patients overall (MITT population: total N = 34)
| AEs . | No. (%) . |
|---|---|
| No. with any treatment-related AEs | 18 (52.9) |
| Chills | 11 (32.4) |
| Pyrexia | 6 (17.6) |
| Flushing | 4 (11.8) |
| Chest discomfort | 3 (8.8) |
| Fatigue | 3 (8.8) |
| Headache | 3 (8.8) |
| Sinus tachycardia | 3 (8.8) |
| Vomiting | 3 (8.8) |
| Anorexia | 2 (5.9) |
| Dyspnea | 2 (5.9) |
| Serum creatinine increased | 2 (5.9) |
| AEs . | No. (%) . |
|---|---|
| No. with any treatment-related AEs | 18 (52.9) |
| Chills | 11 (32.4) |
| Pyrexia | 6 (17.6) |
| Flushing | 4 (11.8) |
| Chest discomfort | 3 (8.8) |
| Fatigue | 3 (8.8) |
| Headache | 3 (8.8) |
| Sinus tachycardia | 3 (8.8) |
| Vomiting | 3 (8.8) |
| Anorexia | 2 (5.9) |
| Dyspnea | 2 (5.9) |
| Serum creatinine increased | 2 (5.9) |
MITT indicates modified intent to treat.
Overall, there were 10 (29.4%) patients who developed an infection during the course of therapy, including 7 patients who had grade 3 or 4 infections assessed as unrelated to elotuzumab. There were no opportunistic viral or fungal infections. The analysis of infection AEs and serious AEs did not reveal a dose-dependent relationship to elotuzumab.
Four patients died during the course of the study. One patient died of renal failure 20 days after the last elotuzumab dose and 13 days after discontinuation from study because of disease progression. Three patients died because of disease progression. The events leading to death were assessed as not related to study drug.
Before the implementation of the revised infusion management guidelines, 13 of 25 treated patients experienced infusion reactions, which with one exception (grade 3 hypersensitivity reaction) were grade 1 or 2 in severity. In total, 5 patients had at least one infusion interrupted, discontinued, or rate of infusion reduced in response to an infusion reaction. Most frequent infusion reactions presented as chills, pyrexia, headache, and flushing. Twenty (58.8%) patients reported an infusion reaction during the first elotuzumab infusion. However, 10 patients had reactions at subsequent infusions. Following a protocol amendment to require infusion reaction premedication immediately before a first dose of elotuzumab, no further serious or grade 3 and 4 infusion reactions were observed. Grade 1 and 2 infusion reactions either resolved spontaneously, typically within 24 hours, or were managed as clinically indicated.
Efficacy
Response.
Overall, no objective responses were observed during the course of the study while patients were receiving treat-ment. Using the EBMT myeloma response criteria, 9 patients (26.5%) were classified as having stable disease; the remaining patients had PD.
PK, pharmacodynamics, and immunogenicity
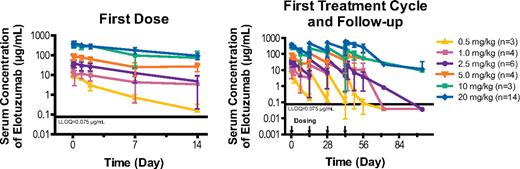
PK parameters after the first and fourth doses are summarized in Table 4. Observed mean drug concentration versus time profiles for all dose groups are shown in Figure 1.
PK parameters for the first and fourth doses of elotuzumab*
| Dose, mg/kg . | Dose . | T1/2λ, days† . | Tmax, hr . | Cmax, mcg/mL‡ . | Vz, mL† . | CL, mL/hr† . | AUCτ, mcg*hr/mL . | AUCinf, mcg*hr/mL . |
|---|---|---|---|---|---|---|---|---|
| 0.5 | 1 | 2.1 ± 0.5 | 1.9 ± 1.0 | 11.3 ± 3.0 | 4291.2 ± 852.5 | 71.4 ± 19.9 | 596.8 ± 151.5 | 634.1 ± 144.9 |
| 4 | NA | 3.2 ± 2.8 | 7.5 ± 2.5 | NA | NA | NA | NA | |
| 1.0 | 1 | 3.2 ± 0.6 | 1.5 ± 0.0 | 17.6 ± 4.8 | 6194.8 ± 2477.9 | 65.2 ± 16.7 | 2328.2 ± 1413.7 | 1342.6 ± 387.0 |
| 4 | NA | 2.0 ± 0.8 | 25.7 ± 19.9 | NA | NA | 5777.5 ± 6395.8 | 1322.0 ± NA | |
| 2.5 | 1 | 4.4 ± 1.8 | 2.8 ± 0.9 | 45.2 ± 12.1 | 4656.3 ± 1932.8 | 40.4 ± 28.3 | 5319.5 ± 2892.3 | 6337.4 ± 3911.5 |
| 4 | NA | 3.5 ± 2.3 | 37.6 ± 24.9 | NA | NA | 5508.9 ± NA | 5884.2 ± 511.9 | |
| 5.0 | 1 | 6.6 ± NA | 4.4 ± 1.7 | 90.5 ± 27.1 | 4757.7 ± 240.9 | 22.1 ± NA | 14 403.9 ± 4086.1 | 13 718.0 ± NA |
| 4 | NA | 4.2 ± 2.1 | 160.7 ± 58.9 | NA | NA | 46 420.5 ± NA | 14 172.8 ± NA | |
| 10 | 1 | 4.6 ± 0.1 | 4.2 ± 2.4 | 337.4 ± 65.8 | 2976.9 ± 366.5 | 15.3 ± 8.6 | 40 544.4 ± 28 741.7 | 28 378.2 ± 12 432.9 |
| 4 | NA | 4.8 ± 1.4 | 216.6 ± 37.2 | NA | NA | 26 506.4 ± NA | 27 220.0 ± NA | |
| 20 | 1 | 7.8 ± 0.3 | 6.8 ± 6.4 | 415.3 ± 90.0 | 4781.6 ± 1482.8 | 15.7 ± 4.9 | 74 850.6 ± 17 969.5 | 86 152.6 ± 26 732.0 |
| 4 | NA | 4.2 ± 1.4 | 563.0 ± 112.5 | NA | NA | 162 390.2 ± 89 871.7 | NA |
| Dose, mg/kg . | Dose . | T1/2λ, days† . | Tmax, hr . | Cmax, mcg/mL‡ . | Vz, mL† . | CL, mL/hr† . | AUCτ, mcg*hr/mL . | AUCinf, mcg*hr/mL . |
|---|---|---|---|---|---|---|---|---|
| 0.5 | 1 | 2.1 ± 0.5 | 1.9 ± 1.0 | 11.3 ± 3.0 | 4291.2 ± 852.5 | 71.4 ± 19.9 | 596.8 ± 151.5 | 634.1 ± 144.9 |
| 4 | NA | 3.2 ± 2.8 | 7.5 ± 2.5 | NA | NA | NA | NA | |
| 1.0 | 1 | 3.2 ± 0.6 | 1.5 ± 0.0 | 17.6 ± 4.8 | 6194.8 ± 2477.9 | 65.2 ± 16.7 | 2328.2 ± 1413.7 | 1342.6 ± 387.0 |
| 4 | NA | 2.0 ± 0.8 | 25.7 ± 19.9 | NA | NA | 5777.5 ± 6395.8 | 1322.0 ± NA | |
| 2.5 | 1 | 4.4 ± 1.8 | 2.8 ± 0.9 | 45.2 ± 12.1 | 4656.3 ± 1932.8 | 40.4 ± 28.3 | 5319.5 ± 2892.3 | 6337.4 ± 3911.5 |
| 4 | NA | 3.5 ± 2.3 | 37.6 ± 24.9 | NA | NA | 5508.9 ± NA | 5884.2 ± 511.9 | |
| 5.0 | 1 | 6.6 ± NA | 4.4 ± 1.7 | 90.5 ± 27.1 | 4757.7 ± 240.9 | 22.1 ± NA | 14 403.9 ± 4086.1 | 13 718.0 ± NA |
| 4 | NA | 4.2 ± 2.1 | 160.7 ± 58.9 | NA | NA | 46 420.5 ± NA | 14 172.8 ± NA | |
| 10 | 1 | 4.6 ± 0.1 | 4.2 ± 2.4 | 337.4 ± 65.8 | 2976.9 ± 366.5 | 15.3 ± 8.6 | 40 544.4 ± 28 741.7 | 28 378.2 ± 12 432.9 |
| 4 | NA | 4.8 ± 1.4 | 216.6 ± 37.2 | NA | NA | 26 506.4 ± NA | 27 220.0 ± NA | |
| 20 | 1 | 7.8 ± 0.3 | 6.8 ± 6.4 | 415.3 ± 90.0 | 4781.6 ± 1482.8 | 15.7 ± 4.9 | 74 850.6 ± 17 969.5 | 86 152.6 ± 26 732.0 |
| 4 | NA | 4.2 ± 1.4 | 563.0 ± 112.5 | NA | NA | 162 390.2 ± 89 871.7 | NA |
Data are mean ± SD.
VZ indicates volume of distribution; and NA, not applicable.
Patients with positive immunogenicity response were excluded from these PK analyses.
Parameter estimation for the fourth dose was excluded as the steady-state concentration was not achieved or data were insufficient for the estimation.
Observed data.
Observed elotuzumab serum concentration versus time curves (mean ± SD). LLOQ indicates lower limit of quantification; and SD, standard deviation.
Observed elotuzumab serum concentration versus time curves (mean ± SD). LLOQ indicates lower limit of quantification; and SD, standard deviation.
After administration of the first dose, Cmax increased in a dose-proportional manner across the dose range of 0.5 mg/kg to 20 mg/kg; however, mean estimates of AUC and AUCinf increased greater than proportionally, suggesting nonlinear PK. The mean clearance decreased and mean T1/2λ increased with an increase in dose from 0.5 mg/kg to 20 mg/kg after first dose administered, indicating a saturation of target-mediated elimination. The volume of distribution was consistent across doses and approximated serum volume.
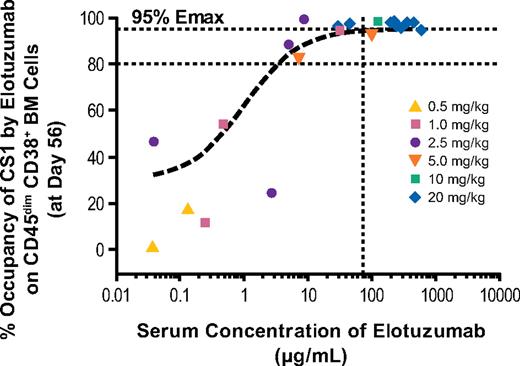
Saturation of CS1 by elotuzumab on bone marrow target cells increased as the dose of elotuzumab increased. At doses of 10 mg/kg and 20 mg/kg elotuzumab, CS1 receptors on bone marrow–derived myeloma cells were consistently saturated. Lower dose groups exhibited more variation in the level of target cell saturation achieved (Figure 2).
CS1 saturation of bone marrow CD38+ cells at day 56. Symbols indicate observed data from individual patients at each dose level. The vertical line indicates target level of serum elotuzumab concentration (70 μg/mL) based on preclinical studies. BM indicates bone marrow; and Emax, maximum effect.
CS1 saturation of bone marrow CD38+ cells at day 56. Symbols indicate observed data from individual patients at each dose level. The vertical line indicates target level of serum elotuzumab concentration (70 μg/mL) based on preclinical studies. BM indicates bone marrow; and Emax, maximum effect.
Table 5 summarizes the incidence of positive results to immunogenicity assays. Overall, 31 patients were tested for immunogenicity; of these, 12 of 31 (39%) were positive for ADAs, and 11 of 12 of these ADA-positive samples were also NAb-positive. The incidence of immunogenicity varied across dose groups (Table 5). Fewer positive responses (3 of 16) were observed for patients in the higher dose groups of 10 mg/kg and 20 mg/kg compared with the lower dose groups (9 of 15). PK analysis suggested that ADA responses seen at lower doses had a more noticeable impact on PK than those seen at higher doses. In the patients with a positive ADA response in the lower dose groups (≤ 5 mg/kg), serum trough concentrations were lower compared with those who had no ADA response. ADA responses were minimized at the 10-mg/kg and 20-mg/kg doses and CS1 receptors on the target cells were saturated, even when an NAb response was positive. There was no correlation between positive ADA results and AEs, response, or disease progression.
Incidence of immunogenicity (patients positive for antidrug antibodies and neutralizing antibodies)
| Dose, mg/kg . | No. . | Confirmed* ADA-positive patients . | NAb-positive patients . |
|---|---|---|---|
| 0.5 | 3 | 3 | 2 |
| 1.0 | 4 | 1 | 1 |
| 2.5 | 5 | 3 | 3 |
| 5.0 | 3 | 2 | 2 |
| 10 | 3 | 1 | 1 |
| 20 | 13 | 2 | 2 |
| Dose, mg/kg . | No. . | Confirmed* ADA-positive patients . | NAb-positive patients . |
|---|---|---|---|
| 0.5 | 3 | 3 | 2 |
| 1.0 | 4 | 1 | 1 |
| 2.5 | 5 | 3 | 3 |
| 5.0 | 3 | 2 | 2 |
| 10 | 3 | 1 | 1 |
| 20 | 13 | 2 | 2 |
Patients having at least 1 postdose sample available for analysis.
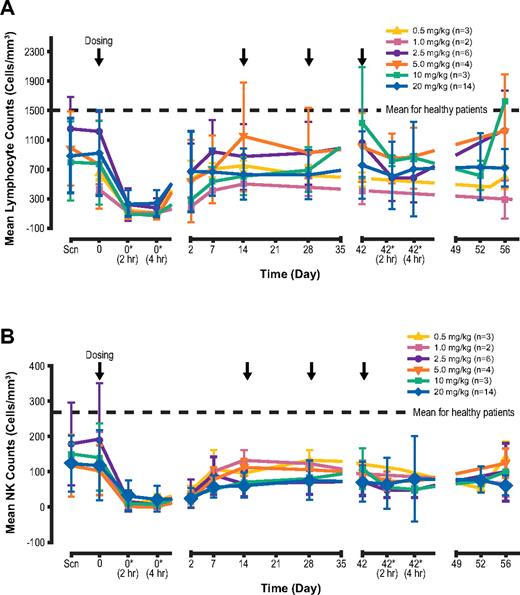
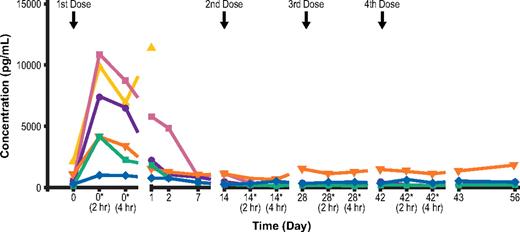
Transient decreases in absolute lymphocyte counts were observed after the first elotuzumab infusion (Figure 3). The transient decrease included both CS1+ (NK cells and CD8+ T cells) and CS1− (CD4+ T cells and B cells) cell subsets, and cell counts subsequently returned to near baseline levels by day 7 of follow-up. This transient reduction in cell counts after the initial dose was associated with a transient increase in IP-10, a chemokine that stimulates migration of activated T cells and NK cells (Figure 4). This suggests that lymphocyte cell trafficking out of the periphery after dosing with elotuzumab was responsible for the decrease observed. There was no evidence of lymphocyte depletion associated with repeated dosing of elotuzumab.
Transient decrease in lymphocyte counts. N = number of patients at baseline. Changes in cell count (from baseline to 2 hours after first dose, and from 2 hours after first dose to day 56) were significant for both (A) total lymphocyte counts (P = .002 and .004, respectively) and (B) NK cell counts (P = .0025 and .032, respectively). *Postdose. All other assessments were performed predose.
Transient decrease in lymphocyte counts. N = number of patients at baseline. Changes in cell count (from baseline to 2 hours after first dose, and from 2 hours after first dose to day 56) were significant for both (A) total lymphocyte counts (P = .002 and .004, respectively) and (B) NK cell counts (P = .0025 and .032, respectively). *Postdose. All other assessments were performed predose.
Individual patient serum IP-10 levels (20-mg/kg cohort, n = 6). *Postdose. All other assessments were performed predose.
Individual patient serum IP-10 levels (20-mg/kg cohort, n = 6). *Postdose. All other assessments were performed predose.
Discussion
Despite success in lymphoma22,23 and leukemia,24,25 effective monoclonal antibody therapy for patients with MM has been elusive to date.26 The earliest trials of monoclonal antibodies in MM involving the anti-CD20 antibody rituximab yielded disappointing results,27 possibly because of relatively low target antigen expression on myeloma cells, or impaired complement-dependent and/or antibody-dependent cellular cytotoxicity (ADCC) in myeloma patients.28 Impaired immunity may also explain the limited success achieved with 2 separate anti-CD40 antibodies (dacetuzumab [SGN-40] and lucatumumab [CHIR12·12, HCD122]) despite more robust target antigen expression.29-31 Monoclonal antibodies directed against other targets relevant to the treatment of MM, including CD56,32 CD38,33 CD138,34 RANKL,35 IL-6,36 and CS1 (described herein) are currently in development.
This study demonstrated that elotuzumab was generally well tolerated at doses sufficient to achieve biologically relevant serum levels and near-complete saturation of the glycoprotein target CS1. Dosing at either 10 mg/kg or 20 mg/kg resulted in target saturation (≥ 95% of CS1 receptors) without dose-limiting toxicity.
With the exception of 1 case of acute renal failure that was attributed to elotuzumab, the treatment-related toxicity observed was largely limited to grade 1 and 2 infusion reactions that usually occurred with the first infusion of elotuzumab, and typically resolved within 24 hours either spontaneously or with treatment as indicated. Infusion reactions are a common side effect of monoclonal antibody therapy. Of note, no grade 3 or 4 and serious elotuzumab-associated infusion reactions were observed after the introduction of a premedication regimen. A refined premedication regimen for prevention of infusion reactions is being tested in subsequent phase 2 and 3 elotuzumab trials.
Although the one case of severe renal impairment was thought to be related to elotuzumab by the treating physician, it is unclear what the mechanism for this might be because CS1 is not detectable in the kidney and elotuzumab is not cleared through the kidney.
As expected based on the lack of tissue expression of CS1, neutropenia and thrombocytopenia were not observed. Infection assessment was of interest in this study because elotuzumab showed transient decreases in the count of several T-cell subsets. There did not appear to be any evidence of chronic depletion of T cells (data not shown). Although there were 7 grade 3 or 4 infections, there did not appear to be a relationship to the dose of elotuzumab. Most infections tended to be bacterial in nature and recovered with standard of care treatment. No evidence of opportunistic infections was observed.
No objective myeloma responses were seen in this heavily pretreated patient population, despite plasma cell target saturation at the higher elotuzumab doses studied. In preclinical models, elotuzumab exerted its anti-myeloma effect primarily via NK cell–mediated ADCC.17 It is conceivable that less-heavily pretreated patients would have better NK cell function, possibly resulting in a more robust ADCC response.37
Both bortezomib and lenalidomide have been shown to enhance elotuzumab-mediated antimyeloma activity in preclinical models.18,19,38 Given this effect and the nonoverlapping toxicity of elotuzumab with either of these agents, there is a strong rationale for exploring combination therapy. Clinical trials combining elotuzumab at doses of 10 mg/kg and 20 mg/kg with each of these drugs are being conducted and have shown evidence of activity.39-41
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the research nurses, physicians, coordinators, and other staff at the study sites; Dr Mohamad A. Hussein for his contributions to the design and execution of this study; Dr Ted Everson, AOI Communications LP, for medical writing assistance in the preparation of this manuscript; and the patients and their families.
This work was supported by Bristol-Myers Squibb. AOI Communications LP provided medical writing, editorial support, and graphics assistance.
Authorship
Contribution: J.A.Z. was a co-investigator for this study, acquired, analyzed, and interpreted data, and drafted, revised, and gave final approval of the manuscript; A.F.M. enrolled subjects, reviewed and corrected data, and revised and interpreted the manuscript; S.S. treated patients and developed and gave final approval of the manuscript; F.v.R. acquired data and drafted and gave final approval of the manuscript; W.I.B. designed the study, analyzed data, and reviewed and gave final approval of the manuscript; H.D. performed the PK and pharmacodynamics analysis for the study, interpreted data, and provided PK/PD figures for the manuscript; J.F. designed the study, analyzed data, and drafted the manuscript; D.E.H.A. designed the clinical study and pharmacodynamic work, and interpreted the PK/pharmacodynamics relationship; and A.K.S. designed and executed the study, including preliminary review of data, and assisted with the preparation and gave final approval of the manuscript.
Conflict-of-interest disclosure: A.F.M. is on the Speakers' Bureau of Genentech Inc, Celgene Corporation, and Millennium Pharmaceuticals Inc. S.S. is on the Speakers' Bureau of Celgene Corporation and Millennium Pharmaceuticals Inc. F.v.R. has received research funding for medical sciences from the University of Arkansas and funding for laboratory research from Facet Biotech (now Abbott Biotherapeutics Corp). W.I.B. has received research funding from PDL Biopharma Inc. H.D. is an employee of Abbott Biotherapeutics Corp (formerly Facet Biotech). J.F. is a former employee of and has previously held stock in Facet Biotech (now Abbott Biotherapeutics Corp). D.E.H.A. is a former employee of Facet Biotech (now Abbott Biotherapeutics Corp). A.K.S. is an employee of Abbott Biotherapeutics Corp (formerly Facet Biotech). J.A.Z. has received speaker honoraria from Celgene Corporation and research funding from Millennium.
Correspondence: Jeffrey A. Zonder, Department of Oncology, Karmanos Cancer Institute, Wayne State University School of Medicine, 4100 John R St, Detroit, MI 48201; e-mail: zonderj@karmanos.org.