Abstract
Red blood cell transfusions have reduced morbidity and mortality for patients with sickle cell disease. Transfusions can lead to erythrocyte alloimmunization, however, with serious complications for the patient including life-threatening delayed hemolytic transfusion reactions and difficulty in finding compatible units, which can cause transfusion delays. In this review, we discuss the risk factors associated with alloimmunization with emphasis on possible mechanisms that can trigger delayed hemolytic transfusion reactions in sickle cell disease, and we describe the challenges in transfusion management of these patients, including opportunities and emerging approaches for minimizing this life-threatening complication.
Introduction
Blood transfusion remains a cornerstone of treatment of patients with sickle cell disease (SCD). Despite improved patient outcomes with hydroxyurea administration, indications for chronic transfusions have increased in the last 10 years and are associated with considerable reduction in morbidity and mortality, most notably in preventing first stroke in children.1-3 However, transfusions can lead to erythrocyte alloimmunization with serious complications for the patient. These antibodies are often directed against antigens expressed on RBCs of white persons, which represent the majority of donors in Western countries.4 Finding compatible units lacking those antigens can sometimes be difficult, and identifying and characterizing the antibodies can be time-consuming and laborious, causing transfusion delays. Genetic as well as acquired patient-related factors are likely to influence the process of alloimmunization.
The most serious consequence of alloimmunization in SCD patients is the risk of developing a delayed hemolytic transfusion reaction (DHTR), which can be life-threatening. In many cases of DHTR in SCD, the patient's hemoglobin level falls below the pretransfusion level, suggesting that, in addition to hemolysis of the transfused RBCs, the patient's own RBCs are lysed, a condition known as hyperhemolysis. Additional transfusions may exacerbate the hemolysis and further worsen the degree of anemia. The destruction of the patient's own RBCs in DHTR of SCD is partly explained by the presence of autoantibodies5 because alloimmunization is known to trigger autoantibody production. However, DHTR/hyperhemolysis cases have also been reported in the absence of detectable alloantibodies or autoantibodies.
In this review, we discuss the known risk factors associated with alloimmunization, then emphasize possible mechanisms that can trigger autoimmunization and DHTR in SCD, and finally describe the challenges in transfusion management of these patients. We emphasize opportunities and emerging approaches for minimizing this life-threatening complication.
RBC alloimmunization pathobiology
Alloimmunization to erythrocytes involves multiple steps, including RBC antigen recognition, processing, and presentation of antigen by HLA class II to TCR, activation of CD4 helper T cells, interaction of T and B cells, and finally B-cell differentiation into plasma cells (Figure 1). Murine and human studies have shown that the process of alloimmunization to RBC antigens can be modulated at each step through acquired and genetic factors, although the relevance of these factors in SCD alloimmunization has not been completely elucidated. Antigenic differences between donor and recipient RBCs are requisite for the initial trigger for alloimmunization. In SCD, multiple studies have shown that alloimmunization risk increases with an increasing number of transfusions.6-11 In addition, women show a higher rate of alloimmunization,11 partially explained by exposure through pregnancy.12
Hypothetical schema of immune response to RBC antigens in alloimmunized versus nonalloimmunized patients. Multiple participants in alloimmunization versus no alloimmunization states are outlined. Preventive interventions and the specific steps in prevention of alloimmunization are shown in yellow. In addition, the possible modes of action of steroids and IVIg for treatment of DHTR are also shown in yellow. The hypothetical model predicts that the chronic inflammatory state in SCD creates a microenvironment with increased inflammatory cytokines, which favors antigen-presenting cells (APCs), such as macrophages and dendritic cells to increase consumption of the transfused allogenic RBCs, and also favor generation of immunogenic peptides in APCs. The person's HLA repertoire will then dictate whether these peptides are presented to the naive CD4+ T helper (Th) cells or not. Alloimmunized sickle patients have increased Th2 frequency, normally associated with humoral immune response, or decreased Treg activity associated with alloimmunization. The skewing toward Th2 or decreased Treg is proposed to be associated with B-cell activation and antibody production. In SCD patients who may have a genetic predisposition to be nonresponders, or patients who do not produce antibodies after allogeneic transfusions, the APCs may be less immunostimulatory such that the peptides presented to the naive CD4 cells are tolerizing and result in the induction of Tregs that can reduce Th2 and/or B cell and APC activation (“?”).
Hypothetical schema of immune response to RBC antigens in alloimmunized versus nonalloimmunized patients. Multiple participants in alloimmunization versus no alloimmunization states are outlined. Preventive interventions and the specific steps in prevention of alloimmunization are shown in yellow. In addition, the possible modes of action of steroids and IVIg for treatment of DHTR are also shown in yellow. The hypothetical model predicts that the chronic inflammatory state in SCD creates a microenvironment with increased inflammatory cytokines, which favors antigen-presenting cells (APCs), such as macrophages and dendritic cells to increase consumption of the transfused allogenic RBCs, and also favor generation of immunogenic peptides in APCs. The person's HLA repertoire will then dictate whether these peptides are presented to the naive CD4+ T helper (Th) cells or not. Alloimmunized sickle patients have increased Th2 frequency, normally associated with humoral immune response, or decreased Treg activity associated with alloimmunization. The skewing toward Th2 or decreased Treg is proposed to be associated with B-cell activation and antibody production. In SCD patients who may have a genetic predisposition to be nonresponders, or patients who do not produce antibodies after allogeneic transfusions, the APCs may be less immunostimulatory such that the peptides presented to the naive CD4 cells are tolerizing and result in the induction of Tregs that can reduce Th2 and/or B cell and APC activation (“?”).
Not all patients develop alloantibodies after exposure to transfused RBCs. This fact pertains not only to patients with SCD but also to all transfused recipients. A recent mathematical modeling study has supported the hypothesis that alloimmunized patients represent a genetically distinct group with an increased susceptibility to RBC sensitization.13 Within this group, only 30% will actually make antibodies, raising the possibility that patient-related factors, including the nature of the underlying disease, may influence alloimmunization in patients with inherited risks. In the following sections, we aim to describe the antigenic RBC determinants in SCD alloimmunization and identify host-susceptibility factors, including those common to any patient population as well as those specific to SCD alloimmunization.
Antigenic differences between donor and recipient RBCs: the initial trigger of alloimmunization
SCD patients are among one of the most frequently alloimmunized transfused population, probably because of polymorphic differences in immunogenic RBC antigens between the predominantly white general blood donors and patients of predominantly African descent. In SCD, the published rate of alloimmunization ranges from 20% to 50%.4,8 However, SCD patients in Uganda and Jamaica, where donors and patients are racially more homogeneous, have reported alloimmunization rates of only 6.1% and 2.6%, respectively,14,15 which are comparable to alloimmunization frequencies reported for the general population of these 2 countries (1%-6%).16 The overall lower use of blood products and blood transfusion therapy for SCD in these countries, in part resulting from concerns about the safety and availability of blood, also may contribute to these lower alloimmunization rates. Prospective comparison of alloimmunization rates per unit transfused between SCD patients in Uganda and Jamaica with those in Western countries will be needed to determine the importance of RBC racial and ethnic differences between donors and recipients on alloimmunization rates. In patients with thalassemia, who are also highly transfused but generally share a more similar ethnic background with blood donors, the rate of alloimmunization is approximately 10%.17 But when the general donor pool is mostly white, Asian patients with thalassemia have an increased rate of alloimmunization compared with white patients.18 Together, these observations support the idea that racial antigenic differences account for increased alloimmunization rates.
Antigenic differences between donors and SCD patients are represented at 3 levels of increasing complexity (Table 1). First, the prevalence of some common but highly immunogenic antigens differs substantially between donors and transfusion recipients. Specifically, C and E in the Rhesus (RH) blood group, K in the Kell (KEL), Fya in the Duffy (FY), Jkb in the Kidd (JK), and S in the MNS blood groups are more frequently encountered in whites than in persons of African descent. Not surprisingly, antibodies against these common antigens are most frequently identified in SCD patients.8
Blood group differences between donors and recipients
| Category . | % in white donors . | % in black recipients . |
|---|---|---|
| Common antigens | ||
| ABO group | ||
| A | 43 | 27 |
| B | 9 | 20 |
| O | 44 | 49 |
| AB | 4 | 4 |
| RH | ||
| D | 85 | 92 |
| C | 68 | 27 |
| E | 29 | 20 |
| c | 80 | 96 |
| e | 98 | 98 |
| KEL | ||
| K | 9 | 2 |
| FY | ||
| Fya | 66 | 10 |
| Fyb | 83 | 23 |
| JK | ||
| Jka | 77 | 92 |
| Jkb | 74 | 49 |
| MNS | ||
| S | 51 | 31 |
| s | 89 | 93 |
| Partial RH antigens | ||
| Partial D among D+ | 1 | 7 |
| Partial C among C+ | 0 | 30 |
| Partial e among e+ | 0 | 2 |
| Low incidence antigens | ||
| VS (RH20) | 0.01 | 26-40 |
| Jsa (KEL6) | 0.01 | 20 |
| Rare blood groups | ||
| U negative (MNS:−5) | 0 | 1 |
| Hrs negative (RH:−18) | 0 | 0.1 |
| HrB negative (RH:−34) | 0 | 0.1 |
| RN (RH:−46) | 0 | 0.1 |
| Jsb negative (KEL:−7) | 0 | 1 |
| Category . | % in white donors . | % in black recipients . |
|---|---|---|
| Common antigens | ||
| ABO group | ||
| A | 43 | 27 |
| B | 9 | 20 |
| O | 44 | 49 |
| AB | 4 | 4 |
| RH | ||
| D | 85 | 92 |
| C | 68 | 27 |
| E | 29 | 20 |
| c | 80 | 96 |
| e | 98 | 98 |
| KEL | ||
| K | 9 | 2 |
| FY | ||
| Fya | 66 | 10 |
| Fyb | 83 | 23 |
| JK | ||
| Jka | 77 | 92 |
| Jkb | 74 | 49 |
| MNS | ||
| S | 51 | 31 |
| s | 89 | 93 |
| Partial RH antigens | ||
| Partial D among D+ | 1 | 7 |
| Partial C among C+ | 0 | 30 |
| Partial e among e+ | 0 | 2 |
| Low incidence antigens | ||
| VS (RH20) | 0.01 | 26-40 |
| Jsa (KEL6) | 0.01 | 20 |
| Rare blood groups | ||
| U negative (MNS:−5) | 0 | 1 |
| Hrs negative (RH:−18) | 0 | 0.1 |
| HrB negative (RH:−34) | 0 | 0.1 |
| RN (RH:−46) | 0 | 0.1 |
| Jsb negative (KEL:−7) | 0 | 1 |
All blood group antigen frequencies have been obtained from The Blood Group Antigen “FactsBook.”114
Matching for E, C, and K reduced the rate of alloimmunization in chronically transfused SCD patients from 3% to 0.5% per unit19 and is now the standard of care in many Western countries, whereas prophylactic extended matching for RH, KEL, FY, JK, and MNS has been shown to be even more effective.19,20 However, there are several problems for this approach, including inventory issues in supplying even the limited RH/KEL-matched units. The most common RH phenotype in SCD patients is D+C−E−c+e+, which is found in less than 2% of whites. To avoid anti-C and anti-E alloimmunization, SCD patients are usually transfused with either units of the same phenotype from donors of African descent, or units with the D−C−E−c+e+ phenotype, which are mainly from white donors. However, 2 problems can arise from the use of such D− units for D+ SCD patients. First, it depletes supply of D− units, which represent less than 15% of all units. These units are needed for transfusing D− persons, especially pregnant females whose fetus is at risk of hemolytic disease of the newborn to prevent anti-D immunization, the most frequent cause of hemolytic disease of the newborn. Second, transfusion of D negative units from white donors, who frequently express other immunogenic common antigens (eg, Fya, Jkb, S; Table 1), can expose black recipients to those other immunogenic RBC antigens and lead to alloimmunization.
It should be also noted that transfusion of RH-compatible units to SCD patients does not totally prevent the risk of alloimmunization because of the presence of numerous RH variants found in persons of African origin. These RH variant antigens account for the second level of antigenic complexity between donor and patient RBCs (Table 1). Within the 5 main RH antigens (D, C, E, c, e), 2 types of variants (“partial” and “weak”) that are encoded by point mutations, multiple missense mutations or hybrid alleles of RHD and RHCE, have been described.21 Patients with partial variants lack some of the epitopes on RH antigens and can make alloantibodies against the missing epitopes when exposed to the complete antigen through transfusion or pregnancy. These alloantibodies can be clinically significant; therefore, persons with partial RH antigens should receive RH antigen-negative RBCs, even though their alloimmunization risk is probably less than patients lacking complete RH antigens.22 In contrast, patients with “weak” antigen variants have quantitatively reduced antigen expression but lack no epitopes so do not routinely become immunized. However, some partial variants may have also a weak antigen expression. For many weak RH antigens, it remains unknown whether patients can make antibodies or not when exposed to the complete antigen.23 With the elucidation of the molecular background of these RH variants in persons of African origin, more information regarding the incidence of these associated antibodies should become available.24
Within D variants, the DAR25 antigen, as well as the DIIIa, DIVa, and some DAU types can lead to alloimmunization.26,27 Some of the variants associated with the RHCE gene, such as partial C encoded by (C)ceS and RN, can also induce pathogenic antibodies.28 Amino acid substitutions that cause loss of epitopes in partial RH variants may be also associated with expression of new antigens referred to as “low-incidence” antigens because of their prevalence in the white reference population (Table 1). In blacks, some are highly prevalent, such as the RH20 (VS) antigen, encoded by the RHCE*ceS allele,29 which is expressed in approximately 26% to 40% of persons of African origin. VS-negative SCD patients receiving blood from donors of same ethnic background can be potentially immunized to VS, but antibodies against VS are not routinely detected by screening tests because most test RBC reagents do not express VS. Prospective studies are needed to determine the frequency of antibodies to VS, by following the VS antigen status in both donors and recipients using DNA tests to evaluate the risk of VS alloimmunization and to determine whether a prevention strategy is necessary.
Many other low-incidence antigens are described in blacks and have resulted in alloimmunization, such as the RH32 encoded by the RN haplotype and the DAK encoded by DIIIa, DOL, and RN.30,31 Low-incidence antigens also exist in other blood group systems, including Jsa and Cob antigens, which have induced alloantibody production in SCD patients.32,33 Antibodies against low-incidence antigens are generally not detected in routine antibody screening tests because test RBCs do not express these antigens. However, they can be detected when a pretransfusion cross-match is performed between samples of RBC to be transfused and patient serum.
The third level of antigenic complexity between SCD patient and donor RBCs that can account for increased SCD alloimmunization rates arises when the recipient lacks an antigen that is expressed in almost all donor RBCs, otherwise referred to as a “high incidence” antigen. Persons with such rare blood groups are at increased risk of alloimmunization because of the high prevalence of the missing antigen within the donor population. The main rare blood types in SCD are in the RH (absence of HrS, HrB, or RH46), KEL (absence of Jsb) and MNS (absence of U) blood groups (Table 1), antibodies against which have been shown to cause RBC destruction.34 Transfusion management for these patients, especially once alloimmunization has occurred, can be extremely challenging. This scenario is best illustrated by the U-negative phenotype of the MNS blood group, which is found in at least 1% of the SCD patients, depending on their country of origin. Low blood donation rates as well as poor donor eligibility resulting from higher prevalence of infectious markers in persons from African countries contribute to the low supply of U-negative and other rare blood types in blacks. Because of the short blood supply in all Western countries, U-negative blood is mainly reserved for patients who have already formed anti-U alloantibodies. For patients with rare RH blood groups, such as HrS, HrB, or RH46-negative phenotypes, the situation is even more complex because few facilities have the corresponding antigen-negative supply.
Individual-specific susceptibility factors
Similar to its role in platelet antigen specific alloimmunization,35 the HLA II genotype of the patient is a key predictor of a person's response to RBC antigens and is likely to influence predisposition to the RBC antibody responder status.36,37 For example, in whites antibody formation to the RBC-specific Fya antigen is strongly associated with the DRB1 04 and DRB1 15 alleles, analogous to observations that HLA-DRB3*0101 and HLA-DQB1*0201 are the key players for platelet antigen HPA-1a alloimmunization. Compared with Fya, the erythrocyte K antigen is highly immunogenic, probably because the potential K antigen–derived peptides can bind to multiple HLA molecules, as indicated by the wide variety of HLA II phenotypes found in persons producing anti-K.36 The HLA-DRB1*1503 allele has been associated with an increased risk of RBC alloimmunization, regardless of the antibody specificity, whereas HLA-DRB1*0901 appears to confer protection against alloimmunization.38 These latter data suggest that, beyond the direct link between HLA II and antibody specificity, HLA alleles may also modulate alloimmunization at a non–antigen-specific level.
Stimulation of helper T cells requires the interaction of peptides presented by HLA II molecules and the cognate TCR on circulating T lymphocytes (Figure 1). T-cell activation can be modulated by CD4+ regulatory T cells (Tregs) that are characterized by coexpression of CD25 and FoxP3. Data from mouse models indicate that Tregs inhibit the magnitude and frequency of alloimmunization39 and that antibody responders have weaker Treg activity and therefore are unable to suppress antibody production compared with nonresponders.40 Possible mechanism(s) of Treg-mediated antibody suppression include inhibition of antibody-producing B cells, directly or indirectly via suppression of helper T-cell function.
In a small study of chronically transfused patients with SCD, reduced peripheral Treg suppressive function was found in antibody responders compared with nonresponders.41 If confirmed in larger cohorts, this information may help identify T cell–associated molecular markers that can accurately predict antibody responders. A higher proportion of Th2 cells, known to regulate humoral immunity, were detected in a subset of alloimmunized SCD patients, documenting a skewed Th2 response in a subgroup of SCD antibody responders (Figure 1).41 Because SCD was the only alloimmunized transfused patient population in that study, however, it remains undefined whether Th2 skewing is a unique feature of alloimmunized SCD patients or also applicable to other immune responders. Although no differences were evident in Th1 and Th17 cell frequencies between alloimmunized and nonalloimmunized SCD patients, the proportion of Th17 cells, which drive tissue inflammation42 as well as circulating TGF-β and IL-6, known to promote human Th17 differentiation,43 were higher in transfused SCD cohorts than in healthy race-matched controls,41 consistent with the proinflammatory nature of SCD (see next paragraph).
SCD-specific susceptibility factors
A main feature of SCD is a chronic inflammatory state, even in steady state, as indicated by increased levels of C-reactive protein,44,45 and inflammatory cytokines (IL-1, IL-6, and IFN-γ)46,47 compared with healthy controls, as well as an increased WBC count that is recognized as a risk factor for disease severity.48
In mouse models, certain proinflammatory stimuli enhance alloimmunization,39,49 most likely because of additional transfused RBC consumption by splenic and liver dendritic cells (DCs), which are potent inducers of alloimmunization.50 The recent observation that a previous febrile reaction to platelets was associated with a higher risk of RBC alloimmunization further supports a role for inflammation promoting alloimmunization in humans,51 although no SCD patients were studied. Hendrickson et al reported that sickle mice (Berkeley and Townes), with or without pretreatment with a viral-like inflammatory stimulus, have a similar rate of alloimmunization compared with wild-type animals, concluding that perhaps expression of other modifying genes besides HbS may be responsible for enhanced RBC alloimmunization in SCD.52 Extrapolating these data to humans must be done with caution, however, because there are inherent differences between clinical features of human SCD and available mouse models,53 and only one example of alloimmunization (to HOD antigen) after a single transfusion was studied.52
It is currently unknown whether alloimmunization rates differ depending on the presence or absence of clinical complications of SCD. For example, it remains unclear whether patients during acute vaso-occlusive crisis (VOC) have an altered alloimmunization potential. VOC, compared with steady-state SCD, is associated with increased inflammatory factors, such as neutrophil chemotaxis and monocyte phagocytosis, but also increased production of IL-6 and augmentation of TNF-α production.54,55 The increased inflammatory state in VOC could therefore affect alloimmunization rates. In contrast, transfusion in the absence of inflammation induces antigenic-specific tolerance in mouse models.56
Children with SCD who are chronically transfused might have less inflammation, which could explain their lower rate of alloimmunization.11,57 However, circulating levels of IL-6 were still elevated in a cohort of chronically transfused young SCD patients compared with healthy controls,58 suggesting that the inflammatory state of SCD may continue despite transfusions. Longitudinal studies involving measurements of inflammatory markers are needed to test whether chronic transfusions, especially with concomitant iron chelation, can reduce inflammation and lower the risk of developing alloantibodies.
Another important issue, recently addressed by Verduzco and Nathan, relates to the effects of age at transfusion initiation on alloimmunization rates in SCD.59 Chronic transfusion protocols for prevention of primary stroke typically begin in childhood, but at an older age than children with thalassemia who begin chronic transfusions in infancy. Multiple studies have shown that the number of cumulative transfusions increases the rate of alloimmunization,6-11 but it is not known whether chronic transfusions initiated at an early age can lower alloimmunization rates in SCD, perhaps by inducing immune tolerance.
Besides these acquired factors, identification of genetic markers predictive of immunization in SCD is an important area of investigation. A polymorphism in the immunoregulatory TRIM21 gene, in close proximity to the β-globin locus, was recently shown to be associated with an increased rate of SCD alloimmunization, especially in early childhood.60 In mice lacking TRIM21, no significant increases in alloimmunization frequency against RBC or platelets were observed, although the mouse model did not have SCD.61 Genome-wide association studies and whole exome sequencing of large cohorts of patients with SCD receiving transfusions should facilitate the identification of genetic predictors of alloimmunization, but these studies will only be informative if performed in conjunction with an accurate laboratory phenotype, which requires routine and thorough testing for RBC alloantibodies.
Autoantibody formation after alloimmunization
Development of RBC autoantibodies after alloimmunization occurs in transfused populations without hemoglobinopathies, although the rates are much higher in transfused patients with thalassemia18 and SCD62-66 with a cumulative incidence of approximately 6% to 10%. Possible theories to explain this phenomenon include failure to regulate alloantibody-induced lymphoproliferation, as well as altered processing and presentation of alloantigens to T cells. Using a mouse model of autoimmune hemolytic anemia (AIHA),67-70 a crucial role for Tregs has been identified for dampening the autoantibody response. AIHA may result from an imbalance between pathogenic and regulatory arms of the immune response because antigen-specific IL-10 secreting regulatory T cells can be detected during periods of remission,71 whereas Th1 responses are present during active disease.69 Hall et al have proposed that AIHA arises when antigen presentation changes as a result of altered cytokine environment because of infection or inflammation.70,71 This not only leads to activation of autoaggressive helper T cells but also lack of presentation of protective peptides that induce Tregs, thus tipping the balance from regulatory toward pathogenic autoreactive T cells.
From the clinical perspective, autoantibodies against red cell antigens can be pathologic with shortened RBC survival and can cause hyperhemolysis through DHTR. In a retrospective study, Castellino et al documented autoantibody-mediated hemolysis in 4 of 14 chronically transfused patients who developed detectable autoantibodies.62 In each case featuring hemolysis, IgG and C3 were present on RBCs as detected by the direct antiglobulin test. Cold-reactive IgM autoantibodies of the IgM isotype also occurred but were less pathogenic. Aygun et al also reported possible involvement of autoantibodies in DHTR/hyperhemolysis.64 In some cases, autoantibodies have been prominent and probably responsible for the development of DHTR, with a clinical picture resembling typical AIHA.5,72,73 In these life-threatening cases, the autoantibodies appear as panagglutinins,74,75 making the identification of compatible blood and characterization of underlying alloantibodies even more difficult. Low titers of autoantibodies can be detected with increased sensitivity using enzyme-treated RBCs that enhance RH antibody reactivity. The clinical significance of these RBC autoantibodies, especially if they do not bind complement, is unclear at this time. Their presence does indicate that autoimmunization is underway, however, suggesting that careful serial monitoring of these patients is warranted.
DHTR
The most life-threatening consequence of alloimmunization in SCD is the development of DHTR with hyperhemolysis. This type of hemolytic reaction is unpredictable and potentially under-recognized because its clinical presentation may resemble a VOC.76,77 DHTR usually occurs between 5 and 15 days after a transfusion and is characterized by a marked drop in the hemoglobin level with the destruction of both transfused and autologous RBCs, and exacerbation of SCD symptoms. Profound reticulocytopenia can worsen the degree of anemia and thus lead to additional transfusions, which further exacerbates the process and result in life-threatening anemia.
Main features and hypothesized mechanisms in SCD
Classically in DHTR, alloantibodies have developed against antigens on transfused RBCs. These antibodies are often not detected in the serum during the pretransfusion screening test and therefore presumably result from remote antigenic exposure and waning of alloantibody titers, followed by current immune restimulation.78 This scenario occurs commonly when detailed and longitudinal transfusion records are not maintained for patients with SCD.
In SCD, cases also exist where the newly formed antibodies were not known to be classically hemolytic, but their pathogenicity was possibly mediated by activated effector cells in SCD, such as hyper-reactive macrophages or NK cells with increased FcR expression.79 In most reported cases (supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article), patients harboring these antibodies were transfused because of an acute complication of SCD or during surgery, both associated with inflammatory cytokine release that can potentially activate effector cells. The SCD milieu may also affect the RBC membrane integrity of transfused cells, making sensitized RBCs more susceptible to hemolysis. Phosphatidylserine (PS) exposure on RBCs is significantly increased after incubation of donor RBCs with pretransfusion plasma from SCD patients in crisis compared with steady-state patients.80 PS exposure on donor RBCs can increase their binding to complement81 and also potentiate their destruction by macrophages through PS receptors.
Cases of DHTR also exist where no detectable antibodies are found in the post-transfusion screening test (supplemental Table 1; and see next section). The mechanism of hyperhemolysis without detectable antibodies is poorly understood, and therefore difficult to explain, prevent, or treat. In SCD, the process has been attributed to macrophage activation, bystander hemolysis, reactive hemolysis, and possible continuation of hemolysis of autologous RBCs during painful VOC.74,82 Incubation of stored donor RBCs in the pretransfusion plasma from SCD patients encountering DHTR without detectable antibodies caused the stored RBCs to undergo eryptosis,80 an antibody-independent phenomenon characterized by erythrocyte shrinkage, membrane blebbing, and PS exposure, all classic features of apoptotic death of nucleated cells and observed in aging or damaged erythrocytes.83 Transfused RBCs may undergo eryptosis in the SCD milieu, potentially because of presence of circulating toxic inflammatory factors that preferentially target and bind receptors present on transfused RBCs but not on autologous RBCs. One such candidate is the chemokine binding protein Duffy (FY) expressed on RBCs of white donors, but rarely on RBCs of patients of African origin (Table 1). In sickle mice, HOD RBCs expressing FY antigens were cleared more rapidly than FY-null RBCs,52 suggesting a role for FY protein in hyperhemolytic reactions without detectable antibodies.
Many cases of DHTR present with vaso-occlusive sickle cell symptoms. One possible explanation for this observation is a cytokine storm that normally accompanies transfusion-associated hemolysis,84-86 which can contribute to VOC by inducing adherence of sickled erythrocytes to endothelium, directly or through activation of neutrophils. In support of this theory, VOC occurred after induction of hemolysis in SCD mice and was hindered by inhibitors of inflammatory cytokine receptors.87 Another possibility is that PS exposure, which is increased on autologous RBCs during post-transfusion hemolysis,80 can contribute to VOC because PS-exposed sickle RBCs have increased adhesiveness.88
Clinical and biologic characteristics of the antibodies in DHTR
A literature review of DHTR since 1984 reveals that new antibodies or additional antibodies have been found on post-DHTR screening tests in the majority of cases (50 of 73 cases; supplemental Table 1). The most frequently encountered antibodies were against Fya, Jkb, and S.89-93 In approximately half of the cases, 2 or more antibodies were found, typically in patients who were previously alloimmunized. These observations support the use of RBCs lacking additional immunogenic antigens, against which alloimmunized SCD patients have not yet been immunized, to decrease the incidence of DHTR. In some cases, the patients had a documented history of the offending antibodies, but the information was not known to the transfusion center. This fact highlights the need for well-maintained patient files and communication between centers that provide transfusion support for patients with SCD.
Several antibodies produced in RH variant carriers have also been associated with alloimmunization and DHTR in SCD. Within D variants, it is known that DAR25 antigen as well as the DIIIa, DIVa, and some DAU types can lead to alloimmunization,26,27 although only DAR has been implicated in DHTR.24,94 Some variants associated with the RHCE gene, such as partial C encoded by (C)ceS and RN, can also induce antibodies that have caused DHTR.28 The low-incidence Goa antigen encoded by the partial DIVa has been associated with DHTR in SCD95 as well as low-incidence antigens in other blood group systems, including Jsa and Cob antigens.32,33 Patients with rare Rh blood groups, such as HrS, HrB, or RH46-negative phenotypes have also been involved in serious cases of DHTR.10,94
In 20 of 73 cases, no additional antibodies were identified in the post-DHTR screening tests. These cases included patients without alloantibodies and documented alloimmunized patients who received matched units for those target antigens.96,97 The lack of detectable new antibodies in the post-transfusion screening test is consistent with a nonantibody-dependent mechanism of posttransfusion hemolysis as described in the previous section.
Diagnosis and management of DHTR
The diagnosis of DHTR in SCD is frequently missed because clinical symptoms resembling VOC presentation may occur up to 15 days after transfusion. Therefore, DHTR should be suspected whenever patients develop vaso-occlusive symptoms after a recent transfusion. Besides the classic biologic and clinical symptoms of post-transfusion hemolysis, the diagnosis of DHTR is often characterized by the coincident disappearance of transfused HbA donor RBCs.
Current management of DHTR in SCD remains controversial because the exact mechanisms remain unclear, especially when antibodies cannot be detected, and also because some of the treatments used in DHTR may be deleterious for SCD patients. For example, corticosteroids can reduce antibody-mediated hemolysis but may lead to a rebound phenomenon with an increase of SCD-related symptoms.98 IVIg, also commonly used for DHTR,5,8,9,19 carries a small risk of thromboembolic complications because of hyperviscosity99 ; however, IVIg is effective for DHTR even in cases where no antibody or new antibody is detected,100 probably because of the inhibitory effects of IVIg on the cellular immune response, including inflammation and macrophage phagocytic function associated with SCD (Figure 1). Erythropoiesis-stimulating agents can also be given to reticulocytopenic patients.100 Rituximab may be another potential treatment option, especially when hemolysis occurs in a known immunized patient. In one reported case, rituximab was used prophylactically in an SCD patient with prior DHTR episodes, to prevent recurrence of autoantibodies and appearance of new alloantibodies.5 Rituximab given with steroids and darbopoietin-α was also effective in treating SCD antibody-mediated hemolysis.101 However, the risk-to-benefit ratio has not been defined in this setting, and prospective studies are warranted. In most cases of DHTR, further transfusions should be withheld to avoid exacerbating the ongoing hemolytic reaction. Because withholding transfusions for patients with cerebral vasculopathy could increase the likelihood of stroke, the risks and benefits of additional transfusions should be carefully evaluated for each patient with DHTR.
Transfusion management strategies to prevent alloimmunization and DHTR
Recommendations for clinical care
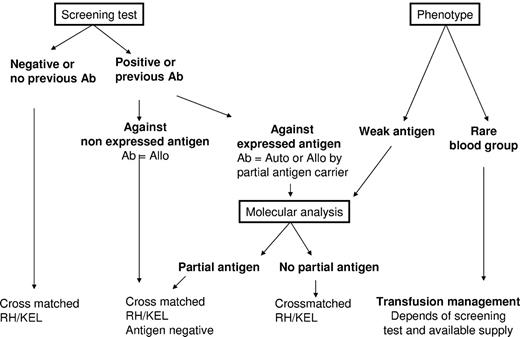
Transfusion of leukoreduced RBC units, which are phenotypically matched for immunogenic RH/KEL blood groups and then cross-matched with a recent serum sample, should be the minimum standard of care for patients with SCD (Figure 2). Despite the associated costs and effort, an additional extended phenotype to other blood groups performed at diagnosis can save valuable time in the transfusion management of patients with multiple alloantibodies and autoantibodies during acute situations. The utility and cost-effectiveness of an early extended phenotype matching have not been reported.
Recommended transfusion strategy for SCD patients. All recommendations are based on the results of the serum antibody screening test results and the extended RBC phenotype of the patient. For patients with no detectable antibodies, no known previous antibodies, and no abnormal RBC phenotype, transfusion of leukoreduced, cross-matched RH/KEL-compatible RBC units is recommended. When antibodies are identified at the screening test or known by history, 2 scenarios can be encountered: (1) The corresponding antigen is not expressed on patient RBCs; therefore, the antibody is an alloantibody (Ab = Allo), and hence antigen-negative RBCs should be transfused. (2) The corresponding antigen is expressed on patient RBCs, and serologic and/or molecular studies are needed to determine whether the antibody is an alloantibody produced in a partial antigen carrier or an autoantibody (Ab = Auto). If an alloantibody, and there are available donor units, compatibility should extend to other common nonexpressed antigens. If an autoantibody, matching for the corresponding antigen is unnecessary. For the phenotype of the patient, 2 situations should be considered: (1) One situation is the presence of a weak antigen, where molecular analysis will be needed to determine whether it is a partial antigen. If the patient has no detectable antibodies, transfusion with an antigen-negative unit is preferred, although antigen-positive RBCs can also be issued because the risk of alloimmunization in patients with weak antigens has not yet been determined. However, close monitoring of possible immunization is needed if antigen-positive units are transfused. (2) Another situation is the presence of a rare phenotype, where availability of high frequency antigen-negative units is the determining factor for the transfusion strategy. If the patient is already immunized against the high-frequency antigen, antigen-negative units are recommended for transfusion. If no rare units are available, the benefit/risk ratio of transfusion has to be considered. For patients who are not yet immunized against high incidence antigens, transfusion of antigen-negative units is also recommended if such units are not in short supply. Otherwise, the standard RBCs for SCD can be issued, and the patients should be monitored closely. Given that antigen-negative units for patients with rare phenotype are by definition in short supply, transfusion management of these patients can become extremely challenging, and we recommend that the risk/benefit ratio of alternative treatments, such as hydroxyurea (hydroxycarbamide) for adult patients or bone marrow or cord blood transplantation for children, is discussed with such patients.
Recommended transfusion strategy for SCD patients. All recommendations are based on the results of the serum antibody screening test results and the extended RBC phenotype of the patient. For patients with no detectable antibodies, no known previous antibodies, and no abnormal RBC phenotype, transfusion of leukoreduced, cross-matched RH/KEL-compatible RBC units is recommended. When antibodies are identified at the screening test or known by history, 2 scenarios can be encountered: (1) The corresponding antigen is not expressed on patient RBCs; therefore, the antibody is an alloantibody (Ab = Allo), and hence antigen-negative RBCs should be transfused. (2) The corresponding antigen is expressed on patient RBCs, and serologic and/or molecular studies are needed to determine whether the antibody is an alloantibody produced in a partial antigen carrier or an autoantibody (Ab = Auto). If an alloantibody, and there are available donor units, compatibility should extend to other common nonexpressed antigens. If an autoantibody, matching for the corresponding antigen is unnecessary. For the phenotype of the patient, 2 situations should be considered: (1) One situation is the presence of a weak antigen, where molecular analysis will be needed to determine whether it is a partial antigen. If the patient has no detectable antibodies, transfusion with an antigen-negative unit is preferred, although antigen-positive RBCs can also be issued because the risk of alloimmunization in patients with weak antigens has not yet been determined. However, close monitoring of possible immunization is needed if antigen-positive units are transfused. (2) Another situation is the presence of a rare phenotype, where availability of high frequency antigen-negative units is the determining factor for the transfusion strategy. If the patient is already immunized against the high-frequency antigen, antigen-negative units are recommended for transfusion. If no rare units are available, the benefit/risk ratio of transfusion has to be considered. For patients who are not yet immunized against high incidence antigens, transfusion of antigen-negative units is also recommended if such units are not in short supply. Otherwise, the standard RBCs for SCD can be issued, and the patients should be monitored closely. Given that antigen-negative units for patients with rare phenotype are by definition in short supply, transfusion management of these patients can become extremely challenging, and we recommend that the risk/benefit ratio of alternative treatments, such as hydroxyurea (hydroxycarbamide) for adult patients or bone marrow or cord blood transplantation for children, is discussed with such patients.
Molecular tools are already available to genotype patients for common antigens, but also for variant antigens and rare blood groups. Such tools are increasingly used in major transfusion reference laboratories. Typing of weak antigens and partial variants by molecular analysis in SCD patients before initiation of transfusion therapy will enable advance preparation of appropriate units for transfusion, especially if the recipient develops an alloantibody and requires further transfusion in an emergency setting. In situations when the SCD patient has developed antibodies against an expressed antigen (such as anti-D in a D-positive patient), prior knowledge of the presence of variants by molecular typing can help distinguish an autoantibody from an alloantibody, which influences the clinical decision about issuing antigen-negative RBC units.
With technologic advances in genomics, high throughput DNA typing platforms will become cheaper for donor typing and should reduce the need for rare serologic reagents to find rare compatible donors.102 This approach will still depend on increasing the pool of donors with African origin, and strategies to promote blood donation in these communities should be an ongoing priority (see “RBC donations from persons of African descent”).
For every transfusion, all known antibodies in the patient's history must be considered to minimize the risk of antibody-mediated DHTR that follows immune restimulation. It is critical to have well-maintained patient records and, if possible, to monitor patients with antibody screening following every transfusion, testing for development of antibodies that may become undetectable before the next transfusion. Based on the study by Schonewille et al,12 posttransfusion screening tests should ideally be performed twice, shortly after transfusion (∼ 3-7 days) and also a longer period of time after transfusion (4-8 weeks) for optimal detection of antibodies. Figure 2 illustrates an algorithm describing the interplay between antibody screening tests and the patient's own RBC phenotype.
RBC donations from persons of African descent
Strategies to increase RBC donations from persons of African descent are critical for tackling the issue of RBC shortage for SCD patients but also should decrease the rate of alloimmunization. Different cultural approaches to blood donation exist among blacks, African-Caribbeans, and whites with a well-documented disparity in donor eligibility depending on ethnicity.103 Some programs, such as the Cooperative Sickle Cell Donor Program, have been launched with the goal of increasing donation from blacks through active recruitment strategies.
Additional problems and questions will arise if black donations grow, however, mainly because a high proportion (up to 10% in the United States) of these donors will have sickle cell trait (SCT). In the majority of countries, including the United States, persons with SCT are eligible for blood donation. It is not known whether SCT blood products can have side effects when transfused into SCD patients, but generally the policy is to avoid transfusion of these blood products into this patient population. From a practical standpoint, transfusing RBC units from SCT donors into SCD patients also confounds routine laboratory monitoring of HbA and HbS levels. Leukoreduced blood products that reduce HLA antibody production, febrile reactions, and CMV transmission, are also problematic for donors with SCT.104 In addition to clogging the filters, leukoreduced SCT units do not always reach accepted standards of less than 1 × 106 leukocytes per unit; these units may escape leukoreduction quality control checks that are performed mostly on random units. Finally, low pH conditions because of citrate anticoagulation can promote HbS polymerization during filtration, thereby increasing adhesion of SCT RBCs to the filters. Attempts to reduce failure of leukoreduction have included a metered anticoagulant device to prevent citrate-mediated HbS polymerization.105
Future strategies to prevent alloimmunization and DHTR
Ongoing studies should explore novel approaches to inhibit alloimmunization in SCD. Immunomodulatory therapies, such as the use of immune cell-depleting agents, costimulatory blockade, and cytokine blockade, may be effective in suppressing alloimmunization (Figure 1), although their use should be carefully balanced against the risk of infections for SCD patients. Rituximab, a mouse-human chimeric antihuman monoclonal antibody that binds CD20 expressed on all B cell, has been used successfully to treat autoantibody production and hemolysis in SCD,101,106 presumably by depleting pathogenic antibody-producing B cells.5 TNF blockade, through the use of neutralizing antibodies to TNF-α, was recently shown to inhibit alloimmunization in a transplant model.107 TNF inhibition has anti-inflammatory effects on multiple pathways, including endothelial activation and leukocyte recruitment, both known to be involved in VOC108,109 and may therefore be effective in SCD for suppression of alloimmunization. Similarly, agonists of adenosine 2A receptors have shown efficacy for treatment of pulmonary inflammation and VOC in SCD mouse models, through inhibition of activation of invariant NK cells and other leukocytes110 and may represent an alternative strategy for limiting alloimmunization by down-regulation of lymphocyte activation. Blockade of costimulatory interactions between T and B cells, for example, by inhibiting the CD40-CD40 ligand pathway with anti-CD40 ligand monoclonal antibody or the B7 pathway with CTLA-4Ig/Abatacept,111 are other possible options. Finally, induction of tolerance through the use of immunodominant peptides derived from the immunogenic polypeptides112 or Treg immunotherapy39 have shown feasibility in mouse studies for inhibition of alloantibody production, some of which are being actively pursued as additional therapeutic approaches for prevention of alloimmunization. Ideally, when genetic modifiers and risk factors become available, transfusion recipients who are genetically predisposed should be carefully matched and monitored to avoid development of alloimmunization.
In conclusion, challenges remain for the diagnosis, prevention, and management of alloimmunization and DHTR in SCD. Understanding the mechanisms and associated risk factors will aid in developing strategies to prevent and inhibit production of antibodies in transfused patients and to minimize its life-threatening complications. Studies in murine models are central to dissection of immune molecular mechanisms of alloimmunization and DHTR. In parallel, careful epidemiologic and prospective studies are needed to investigate critical topics, including the optimal age for initial exposure to RBC antigens and whether alloimmunization rates differ in patients during VOC. Ongoing studies should clarify the role of genetic modifiers of alloimmunization and help identify susceptibility genes that contribute to SCD alloimmunization. Little is known about the etiology of DHTRs without detectable antibodies in SCD, and studies to elucidate their underlying mechanism are critical for SCD patient care. With regard to current transfusion management of SCD patients, we recommend performing an extended phenotype for all patients with SCD at diagnosis, careful monitoring of laboratory tests before and after every transfusion, and a well-maintained electronic system of patient transfusion history. Molecular tools to type most blood group variants have been developed,112,113 and ongoing studies to determine the incidence and clinical significance of antibodies against variants are needed to develop cost-effective genotyping tests for those antigens whose associated antibodies are clinically significant. In parallel, strategies to promote blood donation among persons of African origin should remain a high priority for increasing the donor pool of antigen-matched blood for SCD patients.
Acknowledgments
The authors thank Professor Frederic Galacteros (Sickle Cell Disease Reference Center, Henri Mondor Hospital, Créteil, France) for his helpful comments on the manuscript.
This work was supported in part by the National Heart, Lung, and Blood Institute (grant R21HL097350, K.Y.) and Etablissement Français du Sang.
National Institutes of Health
Authorship
Contribution: K.Y. and F.N.-P. conceived and wrote the manuscript; and R.E.W. edited the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Karina Yazdanbakhsh, Laboratory of Complement Biology, New York Blood Center, 310 East 67th St, New York, NY 10065; e-mail: kyazdanbakhsh@nybloodcenter.org; and France Noizat-Pirenne, Etablissement Francais du Sang Ile de France, 94017 Créteil, France-Inserm, U955, Créteil, 94000, France; e-mail: france.noizat-pirenne@efs.sante.fr.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal