Abstract
Protein S is a cofactor for tissue factor pathway inhibitor (TFPI) that critically reduces the inhibition constant for FXa to below the plasma concentration of TFPI. TFPI Kunitz domain 3 is required for this enhancement to occur. To delineate the molecular mechanism underlying enhancement of TFPI function, in the present study, we produced a panel of Kunitz domain 3 variants of TFPI encompassing all 12 surface-exposed charged residues. Thrombin-generation assays in TFPI-depleted plasma identified a novel variant, TFPI E226Q, which exhibited minimal enhancement by protein S. This was confirmed in purified FXa inhibition assays in which no protein S enhancement of TFPI E226Q was detected. Surface plasmon resonance demonstrated concentration-dependent binding of protein S to wild-type TFPI, but almost no binding to TFPI E226Q. We conclude that the TFPI Kunitz domain 3 residue Glu226 is essential for TFPI enhancement by protein S.
Introduction
Tissue factor pathway inhibitor (TFPI) is a Kunitz-type protease inhibitor consisting of an acidic aminoterminal polypeptide, followed by 3 tandem Kunitz-type domains (Kunitz domains 1, 2, and 3) and a basic carboxyterminal tail.1,2 TFPI exerts its anticoagulant function by inhibiting tissue factor (TF)–induced coagulation in the blood.3-5 Purified assays have shown that FXa inhibition by TFPI occurs in a 2-step process that can be described by the inhibition constants Ki and Ki*,6 respectively, in the following equation:
In 2006, Hackeng et al identified protein S as a cofactor for TFPI that is capable of reducing the Ki for FXa inhibition by TFPI by 10-fold.7 More recently, Ndonwi et al showed that protein S enhancement of TFPI is dependent on the TFPI Kunitz domain 3.8 A TFPI R199L variant showed partial loss of protein S cofactor function compared with wild-type (WT) TFPI.8 The present study is an investigation of the role of all surface-exposed charged residues of TFPI Kunitz domain 3.
Methods
Generation, expression, and purification of TFPI variants
Ten composite and individual point mutations were generated: D194Q/R195Q/R199Q, E202Q/R204Q/K218Q, K213Q/R215Q/K232Q, E226Q/E234Q/R237Q, D194Q, R195Q, R199Q, E226Q, E234Q, and R237Q (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Details of vector construction, protein expression, purification, and quantification can be found in the supplemental Methods.
Thrombin-generation assay determined by CAT
Calibrated automated thrombogram (CAT) was performed in normal or TFPI-depleted plasma (supplemental Methods), as described previously.9,10 Purified or WT TFPI and TFPI variants in concentrated conditioned medium (0-1.5nM) were added to the plasma. Purification status did not influence inhibitory function.
FXa inhibition assay
FXa (0.5nM) activity was monitored by the cleavage of the chromogenic substrate S-2765 (Chromogenix) in the presence of absence of TFPI (0-4nM) and protein S (0-320nM) essentially as described previously.7,8 Curve fitting and determination of the Ki were also as described previously.7 To estimate the concentration of protein S needed to reach 50% of the maximal potentiation of TFPI inhibition of FXa (EC50), the initial velocity of the S-2765 cleavage was plotted against the protein S concentration and the EC50 was determined through fitting of the data to a 1-phase exponential decay nonlinear curve fit.
Binding of protein S to TFPI investigated with SPR
Purified recombinant WT TFPI, TFPI R199Q, and TFPI E226Q were immobilized on a CM5 chip (GE Healthcare) via amine coupling according to the manufacturer's instructions. The immobilization levels were approximately 2500 resonance units. The chip was perfused with purified protein S and measurements were carried out by surface plasmon resonance (SPR) as described previously.8
Results and discussion
Expression of TFPI variants
To investigate the contribution of residues in TFPI Kunitz domain 3 to the protein S enhancement of TFPI, all 12 surface exposed charged residues of the TFPI Kunitz 3 domain were substituted. Composite variants containing 3 substitutions, clustered based on their proximal spatial vicinity, were made (supplemental Figure 1).11 The expression levels of WT TFPI and TFPI variants were similar (4.83-10.0nM) with the exception of TFPI E202Q/R204Q/K218Q, which was expressed at approximately 10-20 times lower levels (approximately 0.56nM). Greater than 87% of TFPI expressed in the conditioned medium was full-length.
Evaluation of TFPI cofactor activity of protein S by CAT
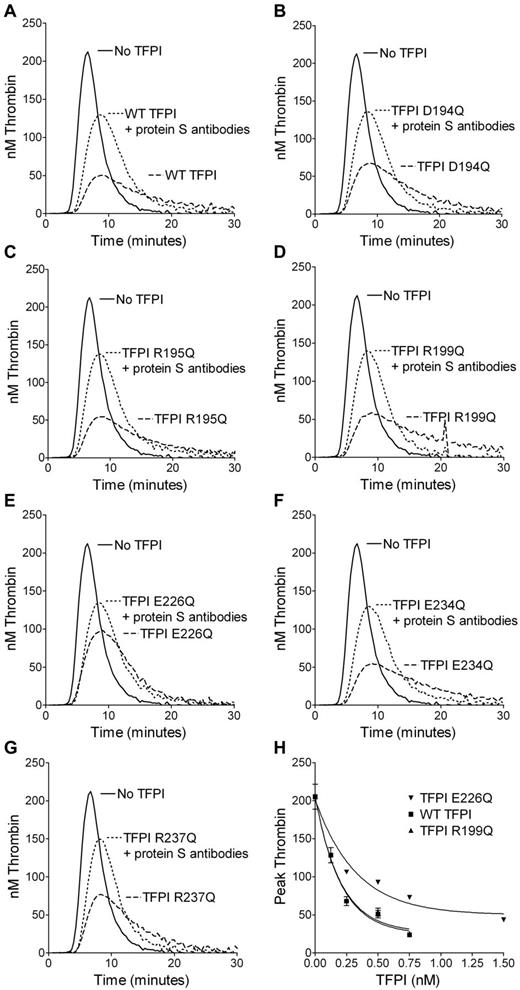
The protein S enhancement of WT TFPI and TFPI variants was evaluated in TFPI-depleted plasma by CAT. TFPI in concentrated conditioned medium (0.25nM) was added to plasma in the presence or absence of inhibitory antibodies against protein S (supplemental Figure 2). Comparing the peak thrombin generation in the presence and absence of antibodies, protein S enhanced WT TFPI by 2.79- ± 0.20-fold (supplemental Table 1). Although TFPI K213Q/R215Q/K232Q was enhanced to a similar level as WT TFPI, TFPI variants E202Q/R204Q/K218Q, D194Q/R195Q/R199Q and E226Q/E234Q/R237Q were appreciably less enhanced by protein S (supplemental Figure 2 and supplemental Table 1). However, TFPI E202Q/R204Q/K218Q had reduced inhibition in the presence of antibodies against protein S; with > 94% being full-length, this reduced function was not attributable to cleavage of TFPI,12-14 but more was likely caused by perturbation of domain folding, as suggested by the greatly reduced expression levels. To elucidate the residues in TFPI D194Q/R195Q/R199Q and TFPI E226Q/E234Q/R237Q that were responsible for the reduced enhancement by protein S, substitutions were studied individually (Figure 1 and supplemental Table 1). The TFPI E226Q variant showed the most striking decrease in protein S enhancement compared with WT TFPI. The decrease in protein S enhancement of TFPI D194Q/R195Q/R199Q could not be fully explained by D194Q, R195Q, or R199Q when substituted individually. Interestingly, the enhancement of TFPI R199Q by protein S was only moderately decreased compared with WT TFPI (2.36- ± 0.10- vs 2.79- ± 0.20-fold) in the thrombin-generation assay.
Inhibition of thrombin generation by TFPI single-point variants in TFPI-depleted plasma. Thrombin generation was measured in TFPI-depleted plasma supplemented with 4μM phospholipids, 1pM TF (Dade Innovin), and 0.25nM TFPI in concentrated conditioned medium in the presence or absence of 2.8μM antibodies against protein S. The inhibition of thrombin generation by WT TFPI (A) was compared with TFPI D194Q (B), TFPI R195Q (C), TFPI R199Q (D), TFPI E226Q (E), TFPI E234Q (F), and TFPI R237Q (G). Representative experiments are shown (n = 3). WT TFPI, TFPI R199Q, and TFPI E226Q were titrated in the absence of antibodies against protein S and the peak thrombin was plotted against the TFPI concentration. Means ± SD (n = 3) are shown in panel H.
Inhibition of thrombin generation by TFPI single-point variants in TFPI-depleted plasma. Thrombin generation was measured in TFPI-depleted plasma supplemented with 4μM phospholipids, 1pM TF (Dade Innovin), and 0.25nM TFPI in concentrated conditioned medium in the presence or absence of 2.8μM antibodies against protein S. The inhibition of thrombin generation by WT TFPI (A) was compared with TFPI D194Q (B), TFPI R195Q (C), TFPI R199Q (D), TFPI E226Q (E), TFPI E234Q (F), and TFPI R237Q (G). Representative experiments are shown (n = 3). WT TFPI, TFPI R199Q, and TFPI E226Q were titrated in the absence of antibodies against protein S and the peak thrombin was plotted against the TFPI concentration. Means ± SD (n = 3) are shown in panel H.
When titrating WT TFPI, TFPI R199Q, and TFPI E226Q in the absence of antibodies against protein S, TFPI R199Q decreased the peak height similar to WT TFPI, whereas TFPI E226Q was appreciably less effective (Figure 1H).
Evaluation of protein S enhancement of TFPI by FXa-inhibition assay
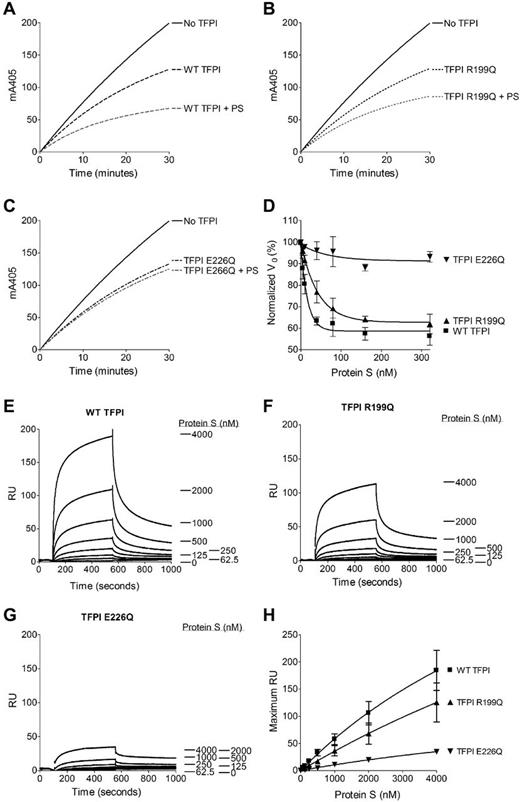
A FXa inhibition assay with purified reagents was used to evaluate quantitatively the protein S enhancement of TFPI E226Q in its inhibition of FXa (Figure 2A-D and supplemental Table 2). TFPI R199Q was also evaluated, because mutation of this residue to leucine has been reported previously to affect protein S enhancement of TFPI.8 Protein S reduced the Ki for WT TFPI by 4.17- ± 3.07-fold. The reduction of the Ki for TFPI R199Q in the presence of protein S was slightly, but not statistically significantly, decreased (3.23- ± 2.02-fold), whereas protein S was unable to decrease the Ki for TFPI E226Q (0.92- ± 0.44-fold). The protein S enhancement of the inhibition of FXa by TFPI can also be studied by determining the EC50 (Figure 2D and supplemental Table 2). Increasing concentrations of protein S enhanced the inhibition of FXa by WT TFPI but, again, no significant effect on FXa inhibition by TFPI E226Q was observed. TFPI R199Q showed a moderate, but not statistically significant, increase in EC50 compared with WT TFPI (31.6 ± 12.1 vs 11.9 ± 2.90nM). Differences in the inhibitory effects presented herein and those reported previously by Ndonwi et al8 may be because of the nature of the residue substitution used in the 2 studies.
Inhibition of FXa by the TFPI variants in the presence or absence of protein S and binding of protein S to TFPI studied by SPR. (A-D) Cleavage of 200μM S-2765 by 0.5nM FXa was monitored at 405nm in the presence of phospholipids (25μM), 2nM WT TFPI (A), TFPI R199Q (B), and TFPI E226Q (C) and in the presence or absence of 100nM protein S (PS). Results from a representative experiment are shown. Increasing concentrations of protein S (0-320nM; Enzyme Research Laboratories) were added to 2nM WT TFPI, TFPI R199Q, and TFPI E226Q. The v0 (initial velocity; expressed as percentage of the v0 in the absence of TFPI) was calculated and plotted against the protein S concentration. Values are given as means ± SD (n = 3) in panel D. (E-H) A CM5 chip was coupled with WT TFPI (E), TFPI R199Q (F), or TFPI E226Q (G) to 2500 resonance units (RU). The flow cells were perfused with increasing concentrations (0-4000nM) of protein S. Results from a representative experiment are shown (n = 3). The maximum RU (RU at 550 seconds of association) was plotted against the protein S concentration. Data are given as means ± SEM (n = 3) in panel H.
Inhibition of FXa by the TFPI variants in the presence or absence of protein S and binding of protein S to TFPI studied by SPR. (A-D) Cleavage of 200μM S-2765 by 0.5nM FXa was monitored at 405nm in the presence of phospholipids (25μM), 2nM WT TFPI (A), TFPI R199Q (B), and TFPI E226Q (C) and in the presence or absence of 100nM protein S (PS). Results from a representative experiment are shown. Increasing concentrations of protein S (0-320nM; Enzyme Research Laboratories) were added to 2nM WT TFPI, TFPI R199Q, and TFPI E226Q. The v0 (initial velocity; expressed as percentage of the v0 in the absence of TFPI) was calculated and plotted against the protein S concentration. Values are given as means ± SD (n = 3) in panel D. (E-H) A CM5 chip was coupled with WT TFPI (E), TFPI R199Q (F), or TFPI E226Q (G) to 2500 resonance units (RU). The flow cells were perfused with increasing concentrations (0-4000nM) of protein S. Results from a representative experiment are shown (n = 3). The maximum RU (RU at 550 seconds of association) was plotted against the protein S concentration. Data are given as means ± SEM (n = 3) in panel H.
Evaluation of binding of protein S to TFPI by SPR
It has been suggested that TFPI and protein S form a complex in plasma, and a direct interaction between TFPI and protein S has been shown using SPR.8,15 Therefore, SPR was performed to elucidate the mechanism responsible for the lack of protein S cofactor activity toward the novel TFPI E226Q variant. As described previously,8,15 protein S bound to WT TFPI in a dose-dependent manner (Figure 2E). Similar to previous studies, no affinity for the TFPI/protein S interaction could be calculated because a 2-phase association was observed and binding curves did not reach equilibrium. Protein S bound to TFPI R199Q (Figure 2F), but apparently less efficiently than to WT TFPI, which is consistent with the trends observed in the functional assays. The TFPI E226Q substitution exhibited severely decreased binding to protein S (Figure 2G-H), which demonstrated the importance of a direct interaction of TFPI and protein S for the functional enhancement of TFPI. We conclude that Glu226 constitutes an essential residue for the interaction of TFPI with protein S involved in the molecular basis of FXa inhibition.
The online version of this article contains a data supplement.
Presented in abstract form at the Nederlandse Vereniging voor Trombose en Hemostase/British Society for Haemostasis and Thrombosis Joint Symposium, Noordwijkerhout, The Netherlands, June 23, 2010.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by the British Heart Foundation (PG/07/044 and PG/09/105).
Authorship
Contribution: J.A., H.M.A., V.H., Y.M., and T.A.J.M performed the experiments; J.A., H.M.A., V.H., J.T.B.C., and D.A.L. designed the research, analyzed the results, and wrote the manuscript; and T.H. contributed vital reagents.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Josefin Ahnström, PhD, or Helena Andersson, PhD, Imperial College London, Centre for Haematology, 5th Floor Commonwealth Building, Hammersmith Hospital Campus, Du Cane Road, W12 0NN, London, United Kingdom; e-mail: j.ahnstrom@imperial.ac.uk or h.andersson06@imperial.ac.uk.
References
Author notes
J.A. and H.M.A. contributed equally to this work.

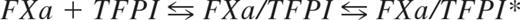
This feature is available to Subscribers Only
Sign In or Create an Account Close Modal