Abstract
Basophils are a rare population of granulocytes that have long been associated with IgE-mediated and Th2-associated allergic diseases. However, the role of basophils in Th17 and/or Th1 diseases has not been reported. In the present study, we report that basophils can be detected in the mucosa of Th17-associated lung and inflammatory bowel disease and accumulate in inflamed colons containing large quantities of IL-33. We also demonstrate that circulating basophils increased memory Th17 responses. Accordingly, IL-3– or IL-33–activated basophils amplified IL-17 release in effector memory T cells (TEM), central memory T cells (TCM), and CCR6+ CD4 T cells. More specifically, basophils promoted the emergence of IL-17+IFN-γ− and IL-17+IFN-γ+, but not IL-17−IFN-γ+ CD4 T cells in TEM and TCM. Mechanistic analysis revealed that the enhancing effect of IL-17 production by basophils in TEM involved the ERK1/2 signaling pathway, occurred in a contact-independent manner, and was partially mediated by histamine via H2 and H4 histamine receptors. The results of the present study reveal a previously unknown function for basophils in augmenting Th17 and Th17/Th1 cytokine expression in memory CD4 T cells. Because basophils accumulated in inflamed inflammatory bowel disease tissues, we propose that these cells are key players in chronic inflammatory disorders beyond Th2.
Introduction
Basophils are the least abundant of the granulocyte population, accounting for only 0.5%-1% of circulating leukocytes, and, together with eosinophils and mast cells (MCs), have long been associated with allergic diseases and helminth infections.1-4 Like MCs, basophils express the tetrameric form of the high-affinity receptor for IgE (FcϵRI) and are a major source of histamine, which is stored in their cytoplasmic basophilic granules. Basophils and MCs belong to distinct cell lineages and are biologically very different.5 Basophils are short-lived circulating cells (estimated half-life of 2 days) that differentiate and mature in the BM, whereas MCs are long-lived, tissue-resident cells that differentiate in peripheral tissues from locally recruited circulating CD34+ precursors released from the BM. MCs are easily detectable at the interface of the organism with the external world; in contrast, basophils are rarely found in normal tissues, but can be detected by immunohistochemistry in inflamed tissues of patients with asthma, allergic rhinitis, and various allergic skin diseases.1,6,7 However, the presence of basophils in the mucosa of patients with inflammatory diseases that are independent of IgE has not been reported.
The CD4 T cells play a key role in orchestrating the pathologic immune reaction of chronic inflammatory disorders. It is now known that Th17 effectors play a crucial role in pulmonary cystic fibrosis (CF).8 Similarly, Th17 and Th1 cells are involved in mucosa-associated chronic disorders such as inflammatory bowel disease (IBD), which include Crohn's disease (CD) and ulcerative colitis (UC).9-11 Specifically, CD is a chronic and relapsing T cell–driven inflammatory disease of the entire gastrointestinal tract. CD4 effector T cells are generated in draining lymph nodes and recruited into the intestinal tissues, where they contribute to the inflammatory process and tissue destruction.9 Once initiated, inflammation is first characterized by the expression of proinflammatory cytokines involved in innate immunity (ie, IL-12, TNF-α, and IL-23), followed by those involved in adaptive immunity (ie, IFN-γ and IL-17).9,12 APC-derived IL-1β, together with IL-6, promotes the development of human Th17 cells in vitro.13 IL-23 promotes the expansion of memory Th17 cells.14 In mice, IL-23 drives pathogenic Th17/Th1 cells.15 Double IL-17- and IFN-γ–producing CD4 T cells are recovered in increased numbers in different models of experimental colitis and, most importantly, in the intestinal mucosa of human IBD patients.11,16-18 In humans, mucosal dendritic cells (DCs) promote Th17/Th1 development.19,20 Overall, defects in the intestinal epithelial barrier function, together with aberrant APC responses to pathogens, have been proposed to participate in the pathogenesis of IBD in genetically susceptible persons.21 To improve therapeutic interventions in chronic inflammatory disorders, it is crucial to understand the mechanisms that result in the recruitment and reactivation of pathogenic Th17 and/or Th1 cells locally. Chemokine-dependent reciprocal interactions take place between neutrophils and Th17, which are colocalized in inflamed CD tissues.22
In the present study, we report that basophils accumulate at inflamed mucosal sites in Th17-associated chronic diseases. We also show that human basophils interact with autologous memory CD4 T cells to increase IL-17 and IFN-γ production. The amplification of Th17 and Th17/Th1 responses, but not Th1 responses, involves basophil-derived histamine and H2 and H4 receptors. Therefore, we propose that human basophils are implicated in inflammatory responses beyond Th2-mediated disorders.
Methods
Human clinical samples
All human subjects signed informed consent forms in accordance with the Declaration of Helsinki and the study was approved by the Institutional Ethic Research Committee (Centre Hospitalier de l'Université de Montréal). Blood was collected in heparinized tubes. Lung fragments were obtained during lung transplantation procedures. Colonic or ileal tissues were obtained from endoscopic biopsies or surgical resection; samples were taken from noninflamed and inflamed gut regions on the basis of clinical, endoscopic, and histologic criteria.
Preparation of lung single-cell suspensions and LPMCs from intestinal mucosa
Lung fragments were digested with collagenase D (Roche) and DNase I (Roche) and dissociated by gentle MACS dissociation. Lamina propria mononuclear cells (LPMCs) were isolated as described.18
Cell purification
Peripheral blood cells were obtained by adapted centrifugation on a Lymphoprep gradient, followed by T-cell depletion by rosetting sheep RBCs. Basophils (CD14−CD1c−FcϵRIhighSIRP-αlow), CD1c+ DCs (CD14−CD1c+FcϵRIlowSIRP-αhigh), and pDCs (CD14−CD1c−FcϵRIlow) were purified from non–T-cell fractions by cell sorting using a FACSAria II cell sorter (BD Biosciences). For morphologic studies, sorted basophils (50 × 103 cells) were cytofuged and stained by the Wright Stain procedure. Effector memory T cells (TEM; CD4+CD8−CD45RA−CD45RO+CD62LlowCD25−), central memory T cells (TCM; CD4+CD8−CD45RA−CD45RO+CD62LhighCD25−), and CCR6+ T cells (CD4+CRTH2−CXCR3−CCR6+) were purified from T cell–enriched fractions. Cell purity was > 99%.
In vitro basophil/T-cell cocultures
TEM (1 × 106/mL), TCM (1 × 106/mL), or CCR6+ T cells (0.5 × 106/mL) were cocultured for 4-5 days in 96-well round-bottom plates with allogeneic or autologous basophils (at a 5:1 ratio) in the presence of IL-2 (100 U/mL; R&D Systems) plus IL-3 (10 ng/mL; R&D Systems), IL-33 (10 ng/mL; Amgen), or thymic stromal lymphopoietin (TSLP; 15 ng/mL; R&D Systems). RPMI 1640 medium with 10% FCS was used for all experiments. For Transwell experiments, TEM and basophils were separated using HTS Transwell 96-well plates (0.4 μm; Corning). TCM (0.5 × 106/mL), TEM (0.5 × 106/mL), or CCR6+ T cells (0.5 × 106/mL) were stimulated with anti-CD3/CD28 beads (Miltenyi Biotec) and cocultured with allogeneic or autologous basophils (0.1 × 106/mL) in the presence of IL-3 or IL-33. For some experiments, TEM or TCM were cocultured with basophils in the presence of diphenhydramine (H1R; SANDOZ), ranitidine (H2R; SANDOZ), JNJ7777120 (H4R Sigma-Aldrich), PD98059 (Sigma-Aldrich), LY294002 (Sigma-Aldrich), and infliximab (Schering-Plough).
Flow cytometry analysis
Whole blood was lysed with ammonium chloride-potassium bicarbonate EDTA lysing buffer (Lonza) for surface markers as described previously.23 Whole blood cell lysate, FACs-sorted purified cells, and mucosal tissues were stained with the mAbs indicated in supplemental Table 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article) in the presence of normal human IgG. For intracytoplasmic staining, cells were restimulated with phorbol 12-myristate 13-acetate/ionomycin (PMA) for 6 hours in the presence of brefeldin A (Calbiochem), fixed, and stained with respective mAbs.
Cytokine production
Histamine (Immunotech), IL-17 (R&D Systems), IL-22 (R&D Systems), IL-33 (Apotech), IFN-γ (Biosource), and TNF-α (Biosource) release were measured by ELISA.
Quantitative real-time PCR
mRNA was extracted from freshly isolated T-cell subsets using RNeasy Plus Mini Kit (QIAGEN) and cDNA was synthesized using SuperScript III First Strand Synthesis SuperMix with random hexamers (Invitrogen) as per the manufacturers' instructions. Real-time quantitative PCR for the human histamine receptors H2 and H4 and for GAPDH was performed using TaqMan Gene Expression Assays and 7500 Real-Time PCR System (Applied Biosystems Life Technologies). The expression of each gene was analyzed as the relative transcript quantification based on the comparative CT (ΔΔCT) method.
Statistical analysis
Statistical analyses were conducted with GraphPad Prism Version 5.0. The Student paired t test or Wilcoxon signed-rank test were used unless otherwise indicated.
Results
Phenotypic analysis of human basophils in the blood and inflamed mucosal tissues of patients with IgE-independent chronic inflammatory diseases
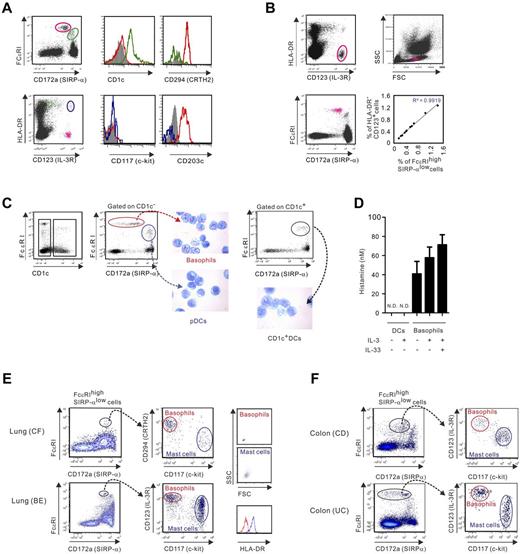
Activated T cells attract basophils to inflammatory lungs in Th2-associated diseases.24,25 We postulated that Th17 and/or Th1 effectors, which infiltrate inflamed mucosal sites in patients with chronic inflammatory diseases that are not associated with Th2,8,18 could also recruit basophils into inflamed tissues. To test this hypothesis, we examined lung and gut tissues of CF and IBD patients, respectively. Before doing so, we chose to identify basophils using a multicolor flow cytometric analysis approach that discriminates FcϵRI+ basophils from the other FcϵRI+ cells, which include DC subsets and mast cells in peripheral blood and tissues, respectively. In whole blood lysate, FcϵRIhighSIRP-αlow cells that were characterized as CD1c− c-kit−CRTH2+CD203c+ cells, were considered identical to basophils conventionally marked as HLA-DR−CD123+ cells (Figure 1A). Indeed, these cells displayed the predicted cell size of basophils and, as expected, the percentages of FcϵRIhighSIRP-αlow and HLADR−CD123+ cells were perfectly correlated (Figure 1B). Morphologic studies on FACS-sorted purified FcϵRI+ cell populations (> 99% cell purity) confirmed that FcϵRIhighSIRP-αlow cells contained typical basophilic granules, whereas FcϵRIlowSIRP-αhigh cells included plasma-like cells (CD1c−) and conventional CD1c+ DCs (Figure 1C). In contrast to DCs, highly purified basophils released histamine in response to IL-3 and/or IL-33, illustrating their functional integrity (Figure 1D).
Phenotypic analysis of human basophils in blood and inflamed mucosal tissues. (A-B) Phenotype of human basophils analyzed in relation to FcϵRI and SIRP-α expression in whole blood lysate. (A) FcϵRIhighSIRP-αlow cells were characterized as HLA-DR−CD123+CD1c−CRTH2+c-kit−CD203c+ (red), FcϵRIlowSIRP-αhigh cells are identified as CRTH2−CD1c+ (green), and HLA-DR+CD123+ cells as c-kit−CD203c− (blue; B). HLA-DR−CD123+ and FcϵRIhighSIRP-αlow cells are shown by forward and side-scatter parameters. Correlation between frequencies of FcϵRIhigh SIRP-αlow cells and HLA-DR−CD123+ cells (n = 11) is shown. (C) Morphologic studies of the purified FcϵRI+ cell subsets using Wright staining. (D) FACS-sorted basophils or CD1c+ DCs were stimulated in the presence or absence of IL-3 (10 ng/mL) with or without IL-33 (10 ng/mL). Histamine release was measured by ELISA after 24 hours. The mean ± SEM for 5 donors is shown. ND indicates not detectable. (E-F) Single-cell suspensions were prepared from the lungs of patients undergoing lung transplantation (E) or the colons of IBD patients (F). Basophils were identified as FcϵRIhighSIRP-αlowc-kit− cells coexpressing CD123 and CRTH2. Forward and side-scatter plot and HLA-DR expression on basophils (c-kit−CRTH2+ or c-kit−CD123+ cells) and mast cells (c-kit+CRTH2− or c-kit+CD123− cells) are examined. Data are representative of 3 CF, 1 bronchiectasis (BE), 3 CD, and 3 UC patients.
Phenotypic analysis of human basophils in blood and inflamed mucosal tissues. (A-B) Phenotype of human basophils analyzed in relation to FcϵRI and SIRP-α expression in whole blood lysate. (A) FcϵRIhighSIRP-αlow cells were characterized as HLA-DR−CD123+CD1c−CRTH2+c-kit−CD203c+ (red), FcϵRIlowSIRP-αhigh cells are identified as CRTH2−CD1c+ (green), and HLA-DR+CD123+ cells as c-kit−CD203c− (blue; B). HLA-DR−CD123+ and FcϵRIhighSIRP-αlow cells are shown by forward and side-scatter parameters. Correlation between frequencies of FcϵRIhigh SIRP-αlow cells and HLA-DR−CD123+ cells (n = 11) is shown. (C) Morphologic studies of the purified FcϵRI+ cell subsets using Wright staining. (D) FACS-sorted basophils or CD1c+ DCs were stimulated in the presence or absence of IL-3 (10 ng/mL) with or without IL-33 (10 ng/mL). Histamine release was measured by ELISA after 24 hours. The mean ± SEM for 5 donors is shown. ND indicates not detectable. (E-F) Single-cell suspensions were prepared from the lungs of patients undergoing lung transplantation (E) or the colons of IBD patients (F). Basophils were identified as FcϵRIhighSIRP-αlowc-kit− cells coexpressing CD123 and CRTH2. Forward and side-scatter plot and HLA-DR expression on basophils (c-kit−CRTH2+ or c-kit−CD123+ cells) and mast cells (c-kit+CRTH2− or c-kit+CD123− cells) are examined. Data are representative of 3 CF, 1 bronchiectasis (BE), 3 CD, and 3 UC patients.
We therefore searched and found FcϵRIhighSIRP-αlow cells in cell suspensions prepared from lung tissue of patients with severe bronchiectasis or CF who had undergone lung transplantation (Figure 1E). In lungs, the FcϵRIhighSIRP-αlow cells were further subdivided into HLA-DR−c-kit−CD123+CRTH2+ cells (basophils) and HLA-DR+c-kit+CD123−CRTH2− cells, which corresponds to the phenotype of mast cells. Using this phenotypic analysis approach, basophils were identified in the LPMCs of patients with the 2 most frequent IBDs, CD and UC (Figure 1F). Our data indicated that basophils are detected in inflamed mucosal tissues in diseases that are associated with Th17, but not related to Th2, and elevated levels of IgE.
Basophils amplify IL-17 production by TEM and TCM CD4 T cells in response to IL-3 or IL-33
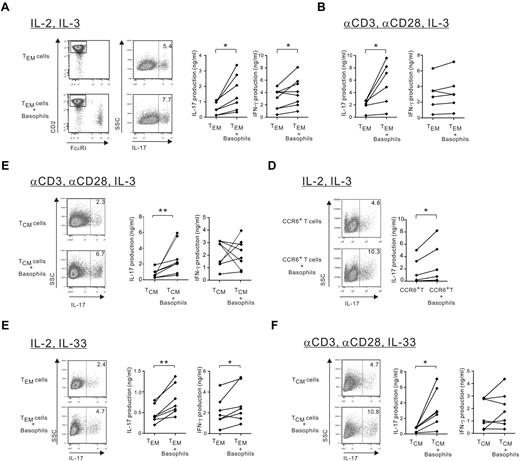
IBDs are associated with Th17, Th1, and Th17/Th1 cells that accumulated in the mucosal inflammatory tissues.11,26 Therefore, we examined whether basophils regulate IL-17 and IFN-γ release by memory T cells. Highly purified basophils significantly augmented IL-17 production in allogeneic and autologous peripheral blood TEM (CD4+CD8−CD45RO+CD45RA−CD62LlowCD25− cells) in the absence of APCs but in the presence of IL-2 and IL-3 (Figure 2A). The CD4 T cell was ascertained as the source of IL-17 release by gating on CD2+FcϵRI− T cells, because phorbol 12-myristate 13-acetate/ionomycin restimulation dampened CD4 expression. TEM represent a minor circulating population of chronically activated T cells by repeated Ag exposure, and we found that basophils maintained their ability to enhance IL-17 production in anti-CD3/CD28–stimulated TEM in the absence of IL-2, whereas TCR stimulation overcame their ability to increase in IFN-γ production (Figure 2B). Furthermore, basophils increased IL-17 but not IFN-γ production by TCR-stimulated TCM (CD4+CD8−CD45RO+CD45RA−CD62LhighCD25− cells; Figure 2C), whereas they augmented both cytokines in the absence of TCR stimulation (supplemental Figure 1A). Memory CCR6+ but not CCR6− CD4 T cells represent the major source of IL-17 and approximately 90% of CCR6+ circulating T cells display the TCM phenotype (supplemental Figure 1B).18 Basophils amplified IL-17 production in allogeneic and autologous IL-2–stimulated CCR6+ T cells in the presence or absence of TCR stimulation (Figure 2D and supplemental Figure 1C). Finally, the augmentation of IL-17 by basophils was also observed in the presence of IL-33 or TSLP, 2 molecules that activate and indirectly promote the survival of basophils27,28 (Figure 1E-F and supplemental Figure 1D). In addition, basophils increased IFN-γ production in IL-2 plus IL-33 TEM but not anti-CD3/anti-CD28–stimulated TCM, confirming data obtained in basophil/memory T-cell cocultures in the presence of IL-3. Based on this result, thereafter, we chose to examine only IL-2–stimulated TEM in the absence of APCs and TCR-stimulated TCM, which mimic memory T-cell activation in mucosal and lymphoid tissues, respectively.
Basophils amplify IL-17 production by effector (TEM) and central memory (TCM) CD4 T cells. TEM, TCM, or CCR6+ CD4 T cells were cultured with or without autologous or allogeneic basophils (at a 5:1 ratio) in the presence of (A-D) IL-3 or IL-33 (E-F). (A) IL-2–stimulated TEM. (B) TEM stimulated by anti-CD3/28–coated beads. (C) TCM stimulated by anti-CD3/28–coated beads. (D) CCR6+ CD4 T cells stimulated by IL-2. (E-F) TEM stimulated by IL-2 and TCM stimulated by anti-CD3/28–coated beads. (A-F) After 4-5 days, the proportion of IL-17–producing CD4 T cells was measured by intracytoplasmic staining (1 representative dot plot) and IL-17 and IFN-γ secretion were measured by ELISA. *P < .05; **P < .01.
Basophils amplify IL-17 production by effector (TEM) and central memory (TCM) CD4 T cells. TEM, TCM, or CCR6+ CD4 T cells were cultured with or without autologous or allogeneic basophils (at a 5:1 ratio) in the presence of (A-D) IL-3 or IL-33 (E-F). (A) IL-2–stimulated TEM. (B) TEM stimulated by anti-CD3/28–coated beads. (C) TCM stimulated by anti-CD3/28–coated beads. (D) CCR6+ CD4 T cells stimulated by IL-2. (E-F) TEM stimulated by IL-2 and TCM stimulated by anti-CD3/28–coated beads. (A-F) After 4-5 days, the proportion of IL-17–producing CD4 T cells was measured by intracytoplasmic staining (1 representative dot plot) and IL-17 and IFN-γ secretion were measured by ELISA. *P < .05; **P < .01.
These results demonstrate that human basophils consistently and reproducibly augment autologous or allogeneic memory Th17 responses in TEM and TCM, regardless of whether the T cells are stimulated via TCR in the presence of IL-3, IL-33, or TSLP in all of the donors examined. However, TCR stimulation appears to overcome the increase of IFN-γ production in both memory T-cell types.
Basophils increase the proportion of IL-17+/IFN-γ+ and IL-17+/IFN-γ− but not IL-17−/ IFN-γ+ cells in TEM and TCM via interference with MAPK pathways
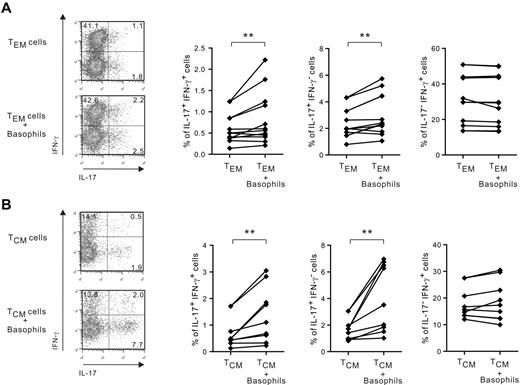
To decipher the differential regulation seen in the increase by basophils of IL-17 and IFN-γ release in relation to TCR stimulation, we examined IL-17 and IFN-γ expression by intracytoplasmic staining. Surprisingly, we found that IL-33– and IL-3–activated basophils did not increase the proportion of single IFN-γ–producing cells in non-TCR–stimulated TEM plus IL-2 and TCR-stimulated TCM cell populations (Figure 3 and supplemental Figure 2), suggesting that they did not modulate Th1 responses. However, basophils significantly augmented the frequency of IL-17+/IFN-γ+ and IL-17+/IFN-γ− CD4 T cells in TEM and TCM. Similar data were obtained in non-TCR–stimulated TCM in the presence of IL-2 and TCR-stimulated TEM cell populations (M.R. and K.W., unpublished observations, July 2012). These data suggest that the increase of IFN-γ secretion by basophils seen in memory CD4 T cells in the absence of TCR stimulation likely reflects their ability to augment Th17/Th1 rather than Th1 responses.
Basophils increase the proportion of IL-17+/IFN-γ+ and IL-17+/IFN-γ− but not IL-17−/ IFN-γ+ cells in TEM and TCM. TEM and TCM cells were cultured with or without basophils in the presence of IL-33. TEM were stimulated by IL-2 (A) and TCM were stimulated by anti-CD3/28–coated beads (B). After 5 days, cells were restimulated with PMA for 6 hours and IL-17 and IFN-γ expression was examined using intracytoplasmic staining. **P < .01.
Basophils increase the proportion of IL-17+/IFN-γ+ and IL-17+/IFN-γ− but not IL-17−/ IFN-γ+ cells in TEM and TCM. TEM and TCM cells were cultured with or without basophils in the presence of IL-33. TEM were stimulated by IL-2 (A) and TCM were stimulated by anti-CD3/28–coated beads (B). After 5 days, cells were restimulated with PMA for 6 hours and IL-17 and IFN-γ expression was examined using intracytoplasmic staining. **P < .01.
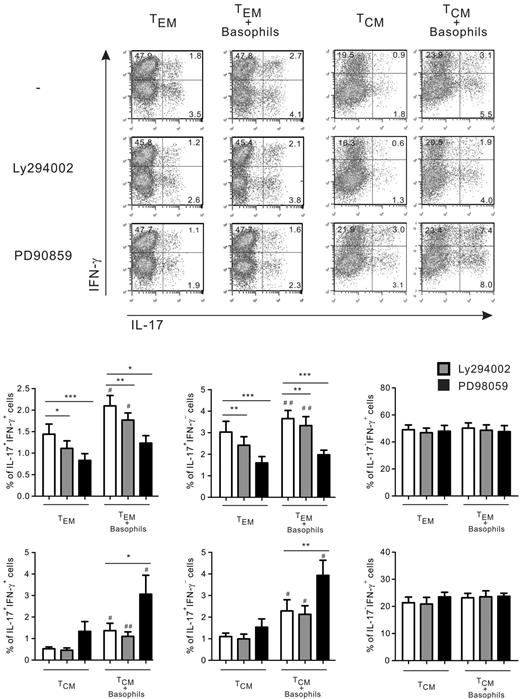
We next sought to explore the molecular pathways that are implicated in the amplification by IL-33–activated basophils of Th17 and Th17/Th1 responses in TEM plus IL-2 and TCR-stimulated TCM cells. Human Th17 cytokine production, and particularly IL-17+/IFN-γ+ and IL-17+/IFN-γ− memory CD4 T cells, is modulated by PI3K signaling pathways, whereas the ERK1/2 pathway down-regulates IL-17 secretion in anti-CD3/anti-CD28–stimulated CD4 T cells via STAT3 inhibition.29,30 As depicted in Figure 4, LY294002 (a PI3K inhibitor) reduced IL-17+/IFN-γ+ and IL-17+/IFN-γ− cells in TEM in the presence or absence of basophils. Furthermore, basophils enhanced Th17 and Th17/Th1 responses in the presence of LY294002. These results suggest that PI3K was not involved in this amplification process by basophils, but corroborate data showing that PI3K signaling downstream of common γ-chain (γc) cytokine receptors augments Th17 responses in CCR6+ CD4 T cells.29 However, the PI3K pathway did not appear to interfere with Th17 and Th17/Th1 responses in TCR-stimulated TCM cultured with or without basophils. In contrast, the ERK pathway differentially regulated Th17 and Th17/Th1 responses in TEM and TCM in the absence of basophils. PD98059 (a MEK kinase inhibitor that inhibits the phosphorylation of ERK1/2) decreased Th17 and Th17/Th1 responses in TEM. However, the proportion of single IL-17– and double IL-17/IFN-γ–producing cells seen in TEM was not significantly increased by basophils in the presence of PD98059, which further amplified the augmentation of IL-17/IFN-γ expression by basophils in TCM. These inhibitors did not modulate the frequency of single IFN-γ–producing CD4 T cells, indicating that they did not adversely alter cellular viability under these experimental conditions.
The MAPK/ERK but not the PI3K pathways are involved in the increase by basophils of IL-17+/IFN-γ− and IL-17+/ IFN-γ+ T cellsin TEM and TCM. TEM stimulated by IL-2 and TCM stimulated by anti-CD3/28–coated beads were cultured in the presence of IL-33 with or without basophils and in the absence or presence of PI3K inhibitor (LY294002 at 2μM) or ERK1/2 inhibitor (PD98059 at 20μM). Shown is intracytoplasmic staining for IL-17 and IFN-γ expression after PMA ionomycin restimulation for 6 hours. Data represent means ± SEM for 6 donors. *,#P < .05; **,##P < .01; ***P < .001 (* indicates cultures ± inhibitors and #, cultures ± basophils).
The MAPK/ERK but not the PI3K pathways are involved in the increase by basophils of IL-17+/IFN-γ− and IL-17+/ IFN-γ+ T cellsin TEM and TCM. TEM stimulated by IL-2 and TCM stimulated by anti-CD3/28–coated beads were cultured in the presence of IL-33 with or without basophils and in the absence or presence of PI3K inhibitor (LY294002 at 2μM) or ERK1/2 inhibitor (PD98059 at 20μM). Shown is intracytoplasmic staining for IL-17 and IFN-γ expression after PMA ionomycin restimulation for 6 hours. Data represent means ± SEM for 6 donors. *,#P < .05; **,##P < .01; ***P < .001 (* indicates cultures ± inhibitors and #, cultures ± basophils).
These results demonstrate that basophils selectively promote the emergence of single IL-17- and double IL-17/IFN-γ–producing cells in TEM and TCM and suggest that the ERK1/2 pathway is involved in this amplification process.
Human peripheral blood basophils amplify Th17 memory responses in a contact-independent manner
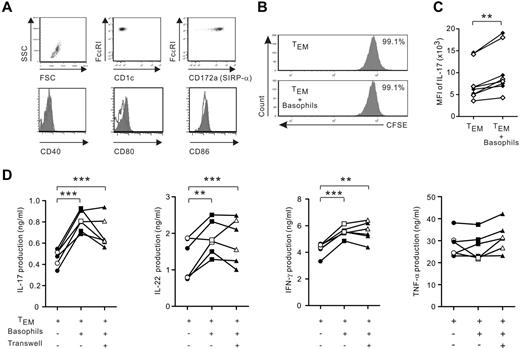
TEM represent the most abundant resident memory T cells in human chronically inflamed mucosal tissues, including inflamed gut tissues of IBD patients.31,32 We therefore further explored the mechanisms that govern the amplification by basophils of Th17 responses in TEM in the absence of TCR stimulation. Highly purified basophils neither expressed detectable costimulatory molecules, including CD40, CD80, and CD86, nor induced proliferation of IL-2–stimulated TEM cells (Figure 5A-B). Furthermore, basophils augmented IL-17 expression at the single cell level, as shown by the increase in mean fluorescence intensity in allogeneic and autologous TEM (Figure 5C). Combined with the absence of detectable MHC class II expression on their cell surface (Figure 1), these data indicate that basophils did not display APC function. We next demonstrated that a direct contact between basophils and TEM was not required to amplify IL-17 production, implicating the participation of soluble molecules (Figure 5D). Finally, basophils not only significantly increased the secretion of IL-17 and IFN-γ in a contact-independent manner, but also augmented IL-22, another cytokine associated with Th17 cells. However, basophils did not facilitate the secretion of TNF-α, a cytokine that has a key function in IBD.9
Basophils amplify cytokine IL-17, IL-22, and IFN-γ release in a contact-independent manner. (A) Expression of costimulatory molecules on purified basophils (CD1c-FcϵRIhighSIRP-αlow); filled histogram represents staining with isotype-matched control mAb. Data from 1 representative experiment of 5 are shown. (B) TEM were labeled with CFSE and cocultured in the presence of IL-2 plus IL-3 with or without basophils. After 5 days, cell proliferation was assessed by CFSE cell dilution. Data from 1 representative experiment of 4 are shown. (C-D) TEM were cocultured with or without autologous (white symbols) or allogeneic (black symbols) basophils (at a 5:1 ratio) in the presence of IL-3 and IL-2. After 4-5 days, cells were restimulated with PMA ionomycin for 6 hours. (C) Mean fluorescence intensity (MFI) of IL-17 expression in IL-17+CD2+FcϵRI− cells. (D) TEM were separated or not from basophils using a Transwell insert. IL-17, IL-22, IFN-γ, and TNF-α secretion were measured with ELISA. *P < .05; **P < .01; ***P < .001.
Basophils amplify cytokine IL-17, IL-22, and IFN-γ release in a contact-independent manner. (A) Expression of costimulatory molecules on purified basophils (CD1c-FcϵRIhighSIRP-αlow); filled histogram represents staining with isotype-matched control mAb. Data from 1 representative experiment of 5 are shown. (B) TEM were labeled with CFSE and cocultured in the presence of IL-2 plus IL-3 with or without basophils. After 5 days, cell proliferation was assessed by CFSE cell dilution. Data from 1 representative experiment of 4 are shown. (C-D) TEM were cocultured with or without autologous (white symbols) or allogeneic (black symbols) basophils (at a 5:1 ratio) in the presence of IL-3 and IL-2. After 4-5 days, cells were restimulated with PMA ionomycin for 6 hours. (C) Mean fluorescence intensity (MFI) of IL-17 expression in IL-17+CD2+FcϵRI− cells. (D) TEM were separated or not from basophils using a Transwell insert. IL-17, IL-22, IFN-γ, and TNF-α secretion were measured with ELISA. *P < .05; **P < .01; ***P < .001.
These data suggest that the proinflammatory activity of basophils is somewhat selective and basophil-derived soluble factors are implicated in this process.
Basophil-derived histamine augments IL-17 production via the H2 and H4 receptors
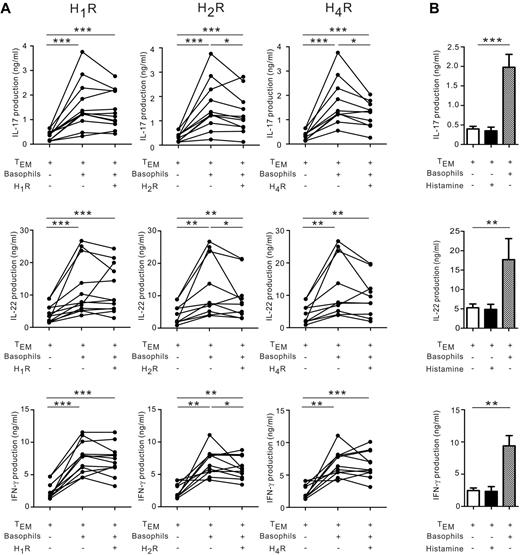
FACS-sorted purified FcϵRIhighSIRP-αlow basophils released histamine (Figure 1D) and histamine reportedly regulated human and murine Th1 and Th17 responses through different histamine receptors (HRs).33 We therefore investigated whether basophil-derived histamine might represent a mechanism by which basophils modulate memory Th17 and Th1 responses. Antagonists to different histamine receptors (ie, H1R, H2R, and H4R) were added to basophil/TEM cocultures (Figure 6). H2R and H4R blockers significantly suppressed IL-17 production (Figure 6A). H4R antagonists tended to decrease IL-22, but not IFN-γ, whereas H2R blockade reduced IL-22 and IFN-γ. In contrast, H1R antagonists did not reduce IL-17, IL-22, or IFN-γ secretion. Basophils could not be substituted by histamine alone to augment Th17 responses, suggesting that histamine is not sufficient and requires a cofactor to amplify Th17 memory responses (Figure 6B).
Histamine receptor antagonist partially blocked IL-17 amplification by basophils. (A) TEM were cultured in the presence of IL-2 plus IL-3 with or without basophils in the presence or absence of antagonists to H1R, H2R or H4R (10−5M). After 4 days, IL-17, IL-22, and IFN-γ secretion was measured using ELISA. (B) TEM were cultured in the presence of IL-2 plus IL-3 with or without 5μM histamine or cocultured with basophils. After 5 days, cytokine production was measured using ELISA. Data represent means ± SEM for 4 donors. *P < .05; **P < .01; ***P < .001.
Histamine receptor antagonist partially blocked IL-17 amplification by basophils. (A) TEM were cultured in the presence of IL-2 plus IL-3 with or without basophils in the presence or absence of antagonists to H1R, H2R or H4R (10−5M). After 4 days, IL-17, IL-22, and IFN-γ secretion was measured using ELISA. (B) TEM were cultured in the presence of IL-2 plus IL-3 with or without 5μM histamine or cocultured with basophils. After 5 days, cytokine production was measured using ELISA. Data represent means ± SEM for 4 donors. *P < .05; **P < .01; ***P < .001.
These data indicate that the augmentation of IL-17 expression by basophils in TEM is partially mediated via the effect of basophil-derived histamine on H2R and H4R, but not H1R.
Basophils and IL-33 are augmented in inflamed mucosal tissues in IBD patients
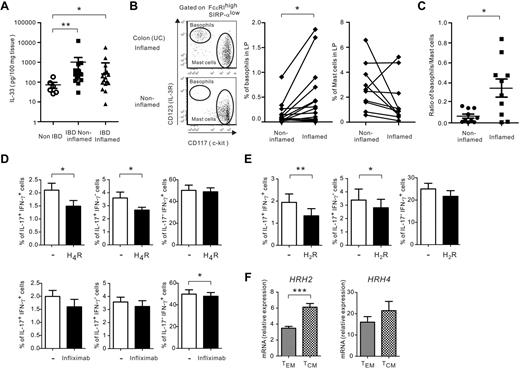
Our present findings indicate that basophils promoted in vitro Th17 responses in the presence of IL-3, IL-33, and TSLP, and could be detected in IBD tissues, a site of accumulation of CCR6+ Th17/Th1 cells.18 To assess the biologic relevance of these in vitro observations, we first measured IL-3, IL-33, and TSLP in colonic explant cultures of IBD and non-IBD patients. IL-33 was produced in large quantities in explant cultures of intestinal mucosa of 16 IBD patients compared with 6 non-IBD patients (Figure 7A). Similar amounts of IL-33 were detected in IBD explants of active and nonactive sites. However, IL-3 and TSLP could not be detected in the same culture supernatants (the detection limits of IL-3 and TSLP are 15 and 30 pg/mL, respectively). We also demonstrated that the frequency of basophils was significantly higher in inflamed compared with symptomless sites in 17 IBD patients (Figure 7B). Because MCs also represent a major source of histamine and thus could exert a similar effect as basophils on memory Th17 responses, we examined the frequency of MCs in the same intestinal tissues. We showed that the frequency of MCs was not increased in inflamed compared with noninflamed gut mucosa in IBD patients (Figure 7B). Furthermore, the basophil/MC ratio was significantly augmented in inflamed mucosa, providing compelling evidence that recruited basophils rather than MCs may be implicated in disease perpetuation (Figure 7C). These data demonstrate that IL-33 is expressed at high levels in IBD mucosa, whereas basophils accumulate in inflamed intestinal tissues.
IL-33 and basophils are augmented in inflamed mucosal tissues in IBD patients. (A) IL-33 release was measured in mucosal explants of patients with IBD or non-IBD. (B) Representative tissue basophil and MC dot plots of inflamed and noninflamed regions of 1 IBD patient (left panels), frequency of basophils (middle panel), and frequency of MCs (right panel) in CD45+ LPMCs isolated from inflamed and noninflamed sites in 17 IBD patients (11 CD and 6 UC patients). (C) Ratio of basophils/MCs in 10 IBD patients (5 CD and 5 UC patients). (D-E) TEM stimulated with IL-2 (D) and TCM stimulated with anti-CD3/28–coated beads (E) were cultured with or without basophils plus IL-33 in the absence or presence of antagonists to H4R (10−5M) or H2R (10−5M), respectively, or infliximab (10 μg/mL). IL-17 and IFN-γ expression was examined using intracytoplasmic staining after PMA ionomycin restimulation for 6 hours. Data represent means ± SEM for 5-8 donors. (F) Quantitative RT-PCR analysis of HRH2 and HRH4 in ex vivo isolated TEM and TCM. Data represent means ± SEM for 5 donors. *P < .05; **P < .01; ***P < .001 as determined by Mann-Whitney U test (A) and paired Student t test or Wilcoxon signed rank test (B-G).
IL-33 and basophils are augmented in inflamed mucosal tissues in IBD patients. (A) IL-33 release was measured in mucosal explants of patients with IBD or non-IBD. (B) Representative tissue basophil and MC dot plots of inflamed and noninflamed regions of 1 IBD patient (left panels), frequency of basophils (middle panel), and frequency of MCs (right panel) in CD45+ LPMCs isolated from inflamed and noninflamed sites in 17 IBD patients (11 CD and 6 UC patients). (C) Ratio of basophils/MCs in 10 IBD patients (5 CD and 5 UC patients). (D-E) TEM stimulated with IL-2 (D) and TCM stimulated with anti-CD3/28–coated beads (E) were cultured with or without basophils plus IL-33 in the absence or presence of antagonists to H4R (10−5M) or H2R (10−5M), respectively, or infliximab (10 μg/mL). IL-17 and IFN-γ expression was examined using intracytoplasmic staining after PMA ionomycin restimulation for 6 hours. Data represent means ± SEM for 5-8 donors. (F) Quantitative RT-PCR analysis of HRH2 and HRH4 in ex vivo isolated TEM and TCM. Data represent means ± SEM for 5 donors. *P < .05; **P < .01; ***P < .001 as determined by Mann-Whitney U test (A) and paired Student t test or Wilcoxon signed rank test (B-G).
To establish a link between IL-33, histamine, and the promotion of Th17/Th1 responses by basophils, we further analyzed the impact of HR blockade on IL-17 and IFN-γ expression at the single-cell level in TEM and TCM (Figure 7D-E and data not shown). We found that H4R and H2R antagonists selectively decreased the frequency of IL-17+/IFN-γ− and IL-17+/IFN-γ+, but not IL-17−/IFN-γ+ cells, in basophil/TEM (Figure 7D and M.R. and K.W. unpublished observations, July 2012), extending our observations seen in IL-3–activated basophils to IL-33 (Figure 6). H2R but not H4R blockade significantly decreased Th17 and Th17/Th1 responses in basophil/TCM cell cocultures (Figure 7E). However, both types of memory CD4 T-cell subsets expressed HRH2 and HRH4 mRNA, whereas TCM tended to express more HR than TEM (Figure 7F). Finally, because chimeric anti–TNF-α (infliximab) is a drug commonly used in the management of IBD patients, we investigated how infliximab modulated the augmentation of Th17 responses in IL-33–activated basophil/TEM cocultures. As depicted in Figure 7D, infliximab, unlike H4R antagonists, did not reduce the frequency of IL-17 and IFN-γ–producing cells in basophil/TEM cocultures. Furthermore, basophils still enhanced IL-17 and IFN-γ release in the presence of infliximab (supplemental Figure 3).
We propose that basophils are recruited in inflamed colons in IBD, a site of abundant IL-33 and exacerbate, at least via histamine, pathogenic memory Th17 responses by promoting the emergence of double IL-17+/IFN-γ+ T cells in the mucosal and systemic immune compartments.
Discussion
The results of the present study revealed an unexpected location and function of human basophils. Basophils infiltrated the inflamed mucosa in patients with chronic inflammatory lung and intestinal diseases associated with Th17 and promoted memory Th17 responses in all of the donors examined. More particularly, basophils augmented the proportion of IL-17+/IFN-γ− and IL-17+/IFN-γ+, but not IL-17−/IFN-γ+ cells in TEM and TCM irrespective of TCR stimulation in the presence of IL-3, IL-33, or TSLP. H2R and H4R blockers inhibited this amplification process, establishing a link between basophils, the cytokines involved in their recruitment and/or activation, histamine, and memory Th17 responses. We therefore propose that basophils may represent previously unknown players in the pathogenesis of chronic inflammatory mucosal diseases that are not associated with Th2 and IgE.
In the earlier studies, human basophils were identified in the skin and airways of allergic patients by immunohistochemistry.5,7 We show herein that basophils were recruited in higher numbers into active compared with nonactive mucosal sites in IBD patients. Although basophils have been detected in situ in the small intestine of parasite-infected mice,34 the mechanisms that attract basophils to lesional tissues remain to be elucidated. Basophils migration to the lymph nodes of parasite-infected or allergen-immunized mice appears to be CD4 T-cell and IL-3 dependent.24,25 IL-33, which we have shown to be produced at high levels in IBD culture explants, is implicated in the expansion of basophils in tissues and can trigger histamine release.27 The overexpression of IL-33 in the intestinal mucosa of UC patients and the increase in the urinary excretion of histamine metabolites in CD patients have been linked to disease severity.35,36 MCs outnumbered basophils at mucosal sites and may thus represent the major source of histamine.37 One report using electronic microscopy showed that basophils seed together with MCs in ileal tissues.38 In the present study, we found that the basophil/MC ratio was significantly elevated in inflamed colons, which reflected the increase in the percentages of basophils but not mast cells. These data suggest that basophils, not mast cell–derived histamine exacerbate inflammation at lesional sites in IBD.
Mechanistic analysis showed that the potentiation of memory Th17 T cells by basophils was not because of increased T-cell proliferation, occurred in a contact-independent manner, and was probably TCR independent because it could be observed in an autologous system without Ag supplementation. Combined with the absence of detectable levels of MHC class II and costimulatory molecules on the surface of circulating and tissue basophils, we largely excluded the possibility that basophils displayed an APC function. However, the possible expression of HLA-DR expression on human basophils is still actively debated.3,23 Therefore, the present study shows that a careful exclusion of contaminating HLA-DR+FcϵRI+ DC subsets (ie, CD1c DCs and pDCs) from preparations of basophils is absolutely required to draw valid conclusions about any potential as-yet recognized function of these rare circulating granulocytes. We show herein that FcϵRIlow DCs can be further discriminated from basophils by their high expression of SIRP-α.
The promotion of IL-17 expression by human basophils occurred in memory CD4 T cells that include TEM, TCM, and CCR6+ but not CCR6− T cells (P.B and K.W., unpublished observations, November 2011), and TCR stimulation did not overcome the amplification of Th17 responses by basophils. IL-17 secretion by CCR6+ T cells was augmented in response to γc cytokine signaling, whereas CCR6− T cells cannot be converted into CCR6+ T cells that produce IL-17.29 We show herein that basophils not only increased in vitro release of IL-17, but also augmented the Th1-associated cytokine IFN-γ in memory T cells. The colonic tissue of patients with CD and UC, 2 distinct entities with overlapping clinical and pathologic characteristics, is enriched in IFN-γ–producing CXCR3+ T cells, IL-17–producing CCR6+ cells, and double IL-17/IFN-γ–producing cells.11,39,40 Therefore, the possible biologic relevance of our present in vitro findings is the accumulation of basophils in inflamed IBD tissues that include CD and UC patients. Although resident TEM represent the most abundant T-cell populations in mucosal tissues, significant proportions of TCM could be also identified.31 Our results further demonstrate that basophils selectively augmented the frequency of IL-17+/IFN-γ− and IL-17+/IFN-γ+ T cells in TEM and TCM. Moreover, basophils did not increase the proportion of IFN-γ–producing CXCR3+CCR6−CRTH2− T cells (K.W. and M.R., unpublished observations, October 2011), further indicating that basophils augmented IFN-γ in Th17 rather than in Th1 cells. Because basophils did not increase TNF-α release by TEM cells, we conclude that the proinflammatory activity of basophils did not induce a generalized activation of memory T cells.
Several soluble factors are known to enhance human Th17 memory responses. PGE2 boosts Th17 through cAMP signaling, whereas IL-7 and IL-15 use the PI3K pathway.41,42 PGE2 synergizes with IL-23 to drive the expansion of human Th17/Th1 cells.43 In fact, IL-23 acts directly on murine intestinal T cells to drive cell proliferation and promote the emergence of IL-23R+ pathogenic IL-17+/IFN-γ− and IL-17+/IFN-γ+, but not IL-17−/IFN-γ+ CD4 T cells, which is reminiscent of the function of basophils reported in the present study.15 However, it is not established that basophils are a source of PGE2, γc inflammatory cytokines, or IL-23. Although the precise mechanisms that govern the ability of basophils to amplify IL-17 production by memory CD4 T cells remain to be fully elucidated, our results suggest that basophils may use the ERK1/2, but not the PI3K or cAMP signaling pathways (M.R. and K.W., unpublished observations, July 2012), to modulate Th17 responses. In fact, these pathways are also implicated directly in HR signaling. In human APCs or MCs, the cytokine suppression by histamine, which is mediated via H2R, involves the cAMP pathway, whereas that mediated via H4R involves the ERK or PI3K pathways.44,45 We have identified basophil-derived histamine as a soluble mediator involved in the process of IL-17 amplification by basophils, because antagonists to H2R and H4R suppressed the enhancing effect by basophils of IL-17 production in TEM, whereas the TCM response appeared to be selectively reduced by H2R blockers, supporting the hypothesis of a differential expression of H2R and H4R on TEM versus TCM. However, we also showed that HRH2 and HRH4 mRNA was expressed on the 2 memory T-cell subsets. H4R is expressed in human Th17 cells and H4R agonists and histamine increase IL-17 but not IL-22 mRNA expression by Th17 cells.46 In contrast, we showed that basophils could not be substituted by histamine alone to augment IL-17, IFN-γ, or IL-22 production, suggesting that histamine acts in concert with an as-yet unidentified soluble cofactor(s) to promote Th17 responses. We conclude that the overall role of histamine and its receptors on human Th cell responses is quite complex and needs to be further clarified.33
Basophils enhanced IL-22 secretion in TEM but not TCM (K.W. and M.R., unpublished observations, March 2012), whereas H4R antagonists reduced IL-17 but not IL-22 production in basophil/TEM cocultures. Although Th17 cells may produce IL-22, Th22 cells that do not produce IL-17 can be generated in vitro, underscoring the dichotomy in the regulation of IL-17 and IL-22 by memory T cells.47 These observations may be therapeutically relevant because IL-22 is considered as guardian of mucosal immunity involved in the defense against gut infections, maintenance of epithelial cell integrity, and secretion of antimicrobial peptides.48 Administration of H4R antagonists ameliorates experimental colitis in rats.49 In contrast to H4R blockade, we show herein that infliximab did not reduce the frequency of pathogenic double IL-17 and IFN-γ–producing CD4 T cells, nor did it overcome the ability of basophils to amplify Th17 responses. In vivo administration of anti–TNF-α to patients results in increase of IL-17 and IFN-γ secretion by in vitro anti–CD3-stimulated PBMCs, and half of the IBD patients are reported to be refractory to anti–TNF-α therapy.50
In conclusion, the results of the present study demonstrate that human basophils accumulate in inflamed IBD tissues and amplify inflammatory memory Th17 cell responses. This suggests that basophils represent previously unknown innate players that, together with eosinophils and neutrophils, are recruited to sites of inflammatory reaction to control the exacerbation of inflammatory responses. Based on these results, we propose that basophil recruitment could also occur in inflammatory diseases that are not associated with Th2, including cancer, infection, and autoimmunity.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Carole Bergeron for her significant contribution to collecting the IBD specimens, the Department of Hematology (Centre Hospitalier de l'Université de Montréal) for technical assistance with the morphologic studies, and Dr H. Mehta for critical reading of the manuscript.
This work was supported by the Canadian Institutes for Health Research, the Crohn Colitis Foundation of Canada, and Allergen. P.B. was supported by the Canadian Allergy, Asthma and Immunology Foundation.
Authorship
Contribution: K.W. and M.S. designed the experiments; K.W., N.B., V.Q.V., P.B., and M.R. performed the experiments; P.F., B.P., R.W., R.L., Y.C., R.T., C.R., and G.S. collected the human intestinal or lung samples and established the diagnoses; and K.W., G.D., and M.S. analyzed the data and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Marika Sarfati, Immunoregulation Laboratory, Centre Hospitalier de l'Université de Montréal, Hôpital Notre-Dame (Pavillon Mailloux, M4211K), 1560 Sherbrooke St E, Montreal, QC H2L 4M1, Canada; e-mail: m.sarfati@umontreal.ca.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal