Abstract
Although a role for oncogenic KIT in driving mast cell disease is clear, the mechanisms driving the multiple phenotypic and clinical manifestations of this disorder are not well elucidated. We now show, using a large cohort of mastocytosis patients, including an almost equal number of aggressive and nonaggressive cases of systemic mastocytosis, that in contrast to the oncogenic KITD816V, TET2 mutation statistically associates with aggressive forms of the disease. By infecting primary murine bone marrow–derived mast cells with KITD816V, we also observe a significant and competitive growth advantage for KITD816V in Tet2-nullizygous compared with wild-type cells. TET2-deficient cells display increased proliferation and can survive in the absence of cytokines. Taken together, these data demonstrate a oncogenic cooperation in mast cells and reveal TET2 mutation as a potential marker to diagnose and predict severe forms of mastocytosis.
Introduction
Mastocytosis is a rare heterogeneous disease characterized by accumulation of morphologically and phenotypically abnormal mast cells in 1 or several organs. A WHO classification describes several subcategories of this disease broadly divided into localized or systemic disease.1 Systemic disease is subsequently divided into indolent and aggressive disease. Aggressive disease is defined by the impairment of at least 1 organ resulting from damage produced by the local mast cell infiltrate (C-finding) and rarely involves the skin.
Mast cell neoplasms are also related to the clonal proliferation of mast cells secondary to a gain of function of KIT, a tyrosine kinase receptor, which results in constitutive signaling.2,3 The majority of adults with mastocytosis present KIT tyrosine kinase domain mutations, most frequently D816V.4
Recent work has provided insight into the causative role of the D816V mutation in differentiation and abnormal clustering of neoplastic cells5 and in induction of mastocytosis.6 Nonetheless, the KITD816V mutation is prevalent in both aggressive and nonaggressive mastocytosis and does not correlate with either form of the disease. Expression of KITD816V alone is unable to transform primary hematopoietic cells.7,8 The clonal nature of mast cell disorders also suggests that KITD816V alone is insufficient to fully transform mast cells in vivo.6 Taken together, the development, some clinical manifestations, and the severity of disease are probably determined by additional mutations.
TET2 is a tumor suppressor gene whose product has been suggested to play an important enzymatic role in DNA demethylation.9-11 Loss-of-function mutations, including mutations affecting the catalytic domain of this protein, have been documented in several different hematopoietic tumors and myeloproliferative neoplasms.12-15
Given the current lack of molecular markers to monitor and predict the clinical course of mastocytosis, we screened a large cohort of mastocytosis patients to determine the prevalence of TET2 mutations and whether these mutations could be associated with KITD816V and aggressive disease.
Methods
Patient data are detailed in supplemental Table 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Mutation analysis for KIT and TET2 has been described.12,16 Lentiviral infection and culture of bone marrow mast cells (BMMCs) have been described.17 Expression analysis was done by standard quantitative real-time PCR using PowerSYBR and a 7500 Fast Real-Time System (Applied Biosystems). Primers are described elsewhere.11 All patients were included in the Mastocytosis Pathophysiological Study, which started in 2003 and is sponsored by the Association for Initiative and Research on Mastocyte and Mastocytosis. The study was approved by Necker Hospital ethical committee and carried out according to the Helsinki convention. Each patient provided informed consent.
Results and discussion
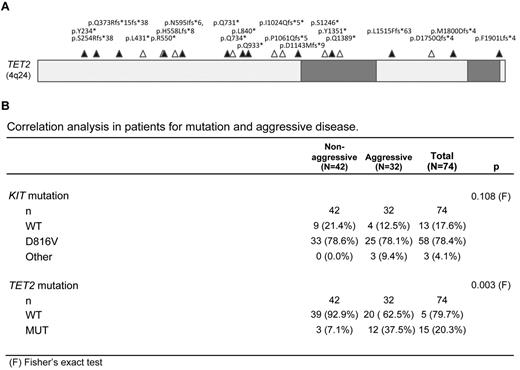
Our cohort consisted of 74 mastocytosis patients, categorized according to the WHO classification for aggressive or nonaggressive disease.7 Consistent with previous studies, the majority of these patients (82.5%) were positive for KIT mutation, 78.4% were positive for KITD816V, and KIT mutation was not correlated with aggressive disease (P = .108; Figure 1). TET2 mutations were detected in 20.3% of patients and were distributed along the gene in a pattern similar to what has been reported in acute myeloid leukemia and chronic myelomonocytic leukemia (Figure 1A).18 Two distinct mutations in the TET2 gene were detected in some patients; and in all cases, TET2 mutation occurred in conjunction with KIT mutation (supplemental Table 1). Importantly, TET2 mutation correlated significantly with aggressive forms of mastocytosis (P = .003; Figure 1). Both TET2 and KIT mutations were found in primary mast cells sorted from patients, and clonal analysis from bone marrow biopsies of 2 patients was also consistent with a model where TET2 mutation is an early event that precedes clonal disease onset of aggressive disease (supplemental Figure 1). Finally, cells from a patient with homozygous TET2 mutation responded normally to tyrosine kinase inhibitors (supplemental Figure 2).
TET2 mutations in human mastocytosis patient cohort. (A) The TET2 gene with conserved regions shaded in gray. Mutations sequenced in patient samples are indicated by triangles (▵). Mutations that were found in combination with at least 1 other TET2 mutation are indicated by a closed triangle (▴). (B) Correlation analysis for mutation and aggressive disease in 74 patients diagnosed with mastocytosis as defined by WHO criteria and enrolled in a prospective national multicenter study 2005-2011.
TET2 mutations in human mastocytosis patient cohort. (A) The TET2 gene with conserved regions shaded in gray. Mutations sequenced in patient samples are indicated by triangles (▵). Mutations that were found in combination with at least 1 other TET2 mutation are indicated by a closed triangle (▴). (B) Correlation analysis for mutation and aggressive disease in 74 patients diagnosed with mastocytosis as defined by WHO criteria and enrolled in a prospective national multicenter study 2005-2011.
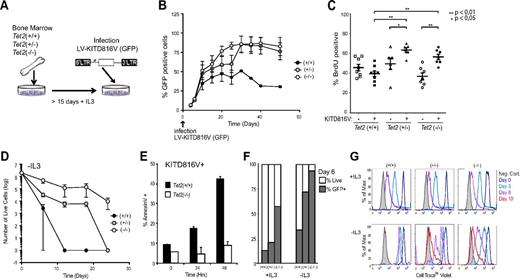
To investigate whether TET2 deficiency and KITD816V could cooperate to transform primary mast cells, we obtained bone marrow from 2 different TET2-deficient mouse lines.19 We then differentiated in vitro bone marrow progenitors from either wild-type or TET2-deficient animals toward the mast cell lineage. All BMMC cultures were homogeneous for mature mast cell markers, including expression of high levels of endogenous murine CD117, FcϵR1a, and mast cell proteases (supplemental Figures 3 and 4). Thus, TET2 deficiency does not appear to overtly corrupt mast cell differentiation.
Strikingly, on infection of these BMMC cultures with lentivirus coding for KITD816V and a GFP tracer (Figure 2A), we observed a rapid and specific selection for KITD816V-positive cells in TET2-deficient cultures (Figure 2B). This result was reproducible in primary mast cells derived from both mouse models and also in BMMCs, infected with KITD816V after 80 days of in vitro culture in the presence of IL-3 (supplemental Figure 5), arguing against a bias resulting from enrichment for infection of stem or progenitor cells whose levels are reportedly higher in the bone marrow TET2-deficient animals.19,20 We also observed a small but significant increase in BrdU incorporation for both Tet2(+/−) and Tet2(−/−) KITD816V-positive populations (Figure 2C), providing an explanation for the competitive growth advantage of these cells.
Ectopic expression of KITD816V in wild-type and TET2-deficient mast cells. (A) Lentiviral infection of in vitro differentiated BMMCs with human KITD816V. (B) Tet2 wild-type (+/+), heterozygous (+/−), and homozygous (−/−) BMMCs were infected with LV-KITD816V at day 0 and GFP expression to detect infected cells over time. n = 6 biologic replicates. (C) Cells were pulse-labeled with BrdU for 24 hours. Quantitation of percent BrdU-positive cells is shown for both GFP/KITD816V-positive and -negative cells. Independent replicates are graphed as points; data are mean ± SEM for each dataset on the graph. Pairwise comparison of datasets was performed using the Mann-Whitney test, and P values are indicated where differences were significant. (D) Cell viability was assessed by trypan blue exclusion and counted using a hemocytometer for Tet2 wild-type (+/+), heterozygous (+/−), and homozygous (−/−) BMMCs infected with KITD816V over time after withdrawal of IL-3 from culture medium. n = 4 biologic replicates. (E) Annexin V staining for samples as in panel D 0-48 hours in the absence of IL-3. (F) Percent GFP-positive cells within total live cell fraction from (D) at day 6 after IL-3 withdrawal. (G) Fluorescence profile of Cell Trace Violet at days 0, 3, 6, and 10 in the presence or absence of IL-3 in labeled BMMCs. Representative profiles are shown for each genotype. Error bars represent SEM of biologic replicates.
Ectopic expression of KITD816V in wild-type and TET2-deficient mast cells. (A) Lentiviral infection of in vitro differentiated BMMCs with human KITD816V. (B) Tet2 wild-type (+/+), heterozygous (+/−), and homozygous (−/−) BMMCs were infected with LV-KITD816V at day 0 and GFP expression to detect infected cells over time. n = 6 biologic replicates. (C) Cells were pulse-labeled with BrdU for 24 hours. Quantitation of percent BrdU-positive cells is shown for both GFP/KITD816V-positive and -negative cells. Independent replicates are graphed as points; data are mean ± SEM for each dataset on the graph. Pairwise comparison of datasets was performed using the Mann-Whitney test, and P values are indicated where differences were significant. (D) Cell viability was assessed by trypan blue exclusion and counted using a hemocytometer for Tet2 wild-type (+/+), heterozygous (+/−), and homozygous (−/−) BMMCs infected with KITD816V over time after withdrawal of IL-3 from culture medium. n = 4 biologic replicates. (E) Annexin V staining for samples as in panel D 0-48 hours in the absence of IL-3. (F) Percent GFP-positive cells within total live cell fraction from (D) at day 6 after IL-3 withdrawal. (G) Fluorescence profile of Cell Trace Violet at days 0, 3, 6, and 10 in the presence or absence of IL-3 in labeled BMMCs. Representative profiles are shown for each genotype. Error bars represent SEM of biologic replicates.
In contrast to wild-type primary BMMCs, TET2-deficient BMMCs infected with KITD816V could survive for > 3 weeks in the absence of IL-3 (Figure 2D-E). Analysis of surviving cells revealed enrichment for KITD816V (Figure 2F) and a capacity for cell division in the absence of IL-3 (Figure 2G). However, unlike BMMCs derived from transgenic KITD816V mice that select for secondary mutations in culture,6 we were unable to render TET2-deficient/KITD816V+ cells completely independent of growth factors within the time frame of these experiments. Overall, our in vitro results are consistent with the transformed phenotype of mast cells in patients; even aggressive forms of systemic mastocytosis are considered to be neoplastic but not malignant.
A high frequency of TET2 mutations has been reported in patients with systemic mastocytosis.15 We now confirm these observations and show a significant correlation between TET2 mutation and aggressive forms of the disease, but, unlike this earlier study, we do not detect an association between TET2 mutation and specific associated clonal hematopoietic nonmast cell lineage disorder. We have been able to observe both TET2 and KITD816V in the primary mast cells from these patients, strongly suggesting an oncogenic cooperation. In vitro analysis indicates that KITD816V induces less apoptosis and more proliferation in TET2-deficient than in wild-type primary cells providing functional support to the human observations.
Overall, our data provide a novel insight into aggressive mast cell disease in patients presenting with TET2 mutations and, importantly, provide a new marker for the diagnosis and treatment of these chronic disorders. At a molecular level, our data also support a model wherein mutations that incur epigenetic deregulation can promote more severe forms of mast cell disease in conjunction with oncogenic activation of KIT. Whether this mechanism also plays a role in other malignant situations with co-occurrence of TET2 inactivation and tyrosine kinase activation, such as in other myeloproliferative neoplasms, needs to be investigated.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by Inserm, la Ligue Nationale Contre le Cancer (postdoc, E.S.; team grant, P.D. and O.A.B.), Agence Nationale de la Recherche–Maladies Rares (P.D. and O.H.), and Institut National du Cancer (P.D. and O.H.).
Authorship
Contribution: E.S. designed and performed experiments and wrote the paper; K.H. processed and sequenced patient samples; T.M. and O.A.B. provided bone marrow from TET2-deficient animals; S.G.-L., G.D., M.O.C., C.L., Y.A., S.L., and O.H. contributed patient data and statistical analysis; P.d.S. and O.A.B. advised on the manuscript; and P.D. supervised all aspects.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Patrice Dubreuil, Centre de Recherche en Cancérologie de Marseille, Inserm U1068, 27 bd Leï Roure, BP 30059, 13273 Marseille Cedex 09, France; e-mail: patrice.dubreuil@inserm.fr.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal