Abstract
An urgent need for new treatment modalities is emerging in elderly patients with acute myeloid leukemia (AML). We hypothesized that targeting VEGF might furnish an effective treatment modality in this population. Elderly patients with AML were randomly assigned in this phase 2 study (n = 171) to receive standard chemotherapy (3 + 7) with or without bevacizumab at a dose of 10 mg/kg intravenously at days 1 and 15. In the second cycle, patients received cytarabine 1000 mg/m2 twice daily on days 1-6 with or without bevacizumab. The complete remission rates in the 2 arms were not different (65%). Event-free survival at 12 months was 33% for the standard arm versus 30% for the bevacizumab arm; at 24 months, it was 22% and 16%, respectively (P = .42). The frequencies of severe adverse events (SAEs) were higher in the bevacizumab arm (n = 63) compared with the control arm (n = 28; P = .043), but the percentages of death or life-threatening SAEs were lower in the bevacizumab arm (60% vs 75% of SAEs). The results of the present study show that the addition of bevacizumab to standard chemotherapy does not improve the therapeutic outcome of older AML patients. This trial is registered as number NTR904 in The Nederlands Trial Register (www.trialregister.nl).
Introduction
The median age at diagnosis of patients with acute myeloid leukemia (AML) is more than 65 years. Progressively higher age independently defines an unfavorable outcome. Although complete remission (CR) can be achieved with several intensive chemotherapy combinations in more than 50% of patients, advancements of response or survival have remained a major investigational challenge.1 Recently, it was shown in AML patients over 60 years of age that escalation of the dose of daunorubicin to twice the conventional dose resulted in a higher response rate from 64% versus 54% in the control standard treatment arm.2 However, only the subgroup of patients between 60 and 65 years of age and those with core-binding factor AML experienced a survival benefit. In a recently published network meta-analysis of 65 randomized clinical trials (15 110 patients) in older patients with AML, most of the amended investigational induction regimens had similar or even worse efficacy profiles compared with the conventional 3 + 7 induction regimen with daunorubicin and cytarabine.3 Median overall survival has at best minimally increased during the last 30 years, most probably reflecting advances in supportive care rather than in antileukemic therapy. There is an urgent need for new treatment modalities in the elderly AML patient group.
VEGF is an essential regulator of physiologic and pathologic angiogenesis, but it also triggers the growth, survival, and migration of leukemia cells.4 Microvessel density is increased in AML compared with normal BM5 and decreases during chemotherapy-induced aplasia in patients who achieve a CR but not in those who fail therapy.6 Recently, it was shown by dynamic MRI that BM angiogenesis is increased in AML patients, with differences seen between individual patients.7 Increased BM angiogenesis was highly correlated with adverse clinical outcome. Others have found that the amount of VEGF produced by AML cells is inversely related to the duration of CR and survival. VEGF-C production by stromal cells, via interaction with VEGF receptor-3, protects AML cells from chemotherapy-induced apoptosis.8 Given these comprehensive scientific data, we assumed that VEGF may act as a growth and survival factor in AML and may contribute to drug resistance. Therefore, we hypothesized that targeting the growth factor VEGF could be a new treatment modality in AML. Bevacizumab is a recombinant humanized IgG mAb directed against all biologic active forms of VEGF because it recognizes the binding sites for its cognate receptors.9 Various trials combining cytostatic drugs with antiangiogenesis therapy have shown an improved outcome in solid tumors, including colon cancer, renal cancer, and glioblastoma.10-12
In a single-arm trial in AML, investigators combined bevacizumab with cytosine-arabinoside–based treatment using timed sequential therapy. In this group of patients with very poor risk AML, a promising response rate of 48% was reported, with 35% of patients still alive after 1 year and 18% still alive after 2 years.13
We report herein the results of a randomized multicenter phase 2 clinical trial in which we compared the addition of bevacizumab with standard chemotherapy in newly diagnosed AML patients 60 years of age or older.
Methods
Patients and eligibility
Previously untreated patients, 60 years of age or older, with a cytologically confirmed diagnosis of de novo AML (not acute promyelocytic leukemia) or with refractory anemia with excess of blasts and an International Prognostic Scoring System (IPSS) score of 1.5 or higher and a World Health Organization performance score of 2 or less were eligible for inclusion. Patients with secondary AML progressing from antecedent myelodysplasia were also eligible. Exclusion criteria included clinically significant cardiovascular disease, including cerebrovascular accidents (≤ 6 months before randomization), myocardial infarction (≤ 6 months before randomization), unstable angina, New York Heart Association grade 2 or greater congestive heart failure, serious cardiac arrhythmia requiring medication, reduced left ventricular ejection fraction of < 50% as evaluated by echocardiogram or MUGA scan, uncontrolled hypertension, proteinuria, major surgical procedure, open biopsy, or significant traumatic injury within 28 days before study treatment start, or anticipation of the need for major surgical procedure during the course of the study. Patients with serious, nonhealing wound, ulcers, or bone fractures or with bleeding diathesis or coagulopathy (unless related to AML) were not eligible. Other standard general medical exclusions were also applied. All patients provided written informed consent.
Risk classifications
Patients were classified into prognostic categories (favorable, intermediate, unfavorable, very unfavorable) on the basis of the karyotype of the leukemic cells. Favorable risk was defined by the presence of abnormalities in core-binding factors and unfavorable risk by the presence of complex cytogenetic abnormalities (at least 3 unrelated cytogenetic abnormalities); monosomies or partial deletions of chromosome 5 or 7 [del(5q), del(7q), −5, −7]; abnormalities of the long arm of chromosome 3 [(q21;q26), t(6;9)(p23;q34), or t(9;22)(q34;q11.2)], or abnormalities involving the long arm of chromosome 11 (11q23) unless the criteria for a monosomal karyotype were fulfilled. The presence of a monosomal karyotype defined the category of very unfavorable prognosis. Any other cytogenetic abnormalities or AML without cytogenetic abnormalities or with loss of an X or Y chromosome as the only abnormality were considered to indicate an intermediate risk.14
Study design
Eligible patients were randomly assigned to receive daunorubicin at a dose of 45 mg/m2 administered intravenously over the course of 3 hours on days 1-3 of the first cycle of induction treatment plus cytarabine at a dose of 200 mg/m2 administered by continuous infusion for 7 days with or without bevacizumab at an initial dosage of 5 mg/kg and eventually at a dose level of 10 mg/kg intravenously for 60 minutes at day 1 and 15. The dosage daunorubicin of 45 mg/m2 was used because this study was launched before the results of the HOVON 43 trial showing that 90 mg/m2 was superior became available.2
In the second cycle of treatment, patients received cytarabine at 1000 mg/m2 twice daily given intravenously over the course of 6 hours on days 1-6 with or without bevacizumab according the schedule and at the dose levels given in the previous paragraph.
Cycle 2 was given as soon as possible after cycle 1, at least within 8 weeks after the start of cycle 1. Patients who developed any grade 4 or nonresolving grade 3 toxicity attributable to bevacizumab were taken off of the drug.
Bevacizumab was provided free of charge by Roche (Basel, Switzerland). The study was divided in 2 parts. The first part (part A) was designed to determine whether the planned dose of 10 mg/kg bevacizumab was tolerable. Therefore, the initial dose of bevacizumab in the first cohort of patients was 5 mg/kg intravenously on days 1 and 15 of each cycle randomized against the control arm. Decisions regarding dose escalation to 10 mg/kg intravenously on days 1 + 15 of each cycle, continuation at dose level 5 mg/kg, or stopping the drug depended on the incidence of dose-limiting toxicities (DLTs; as defined in the next section). In the second part of the study (part B), the safety, tolerability, and efficacy were assessed at the selected dose of bevacizumab. If the upper limit of the 80% confidence interval of the difference (Dcr) in CR rates between the experimental arm at the final dose level and the standard treatment was smaller than 0.10, than the study would be stopped because of inefficacy; otherwise, the study would be continued as a phase 2 or 3 trial.
This trial is registered as number NTR904 in the Nederlands Trial Register (www.trialregister.nl) and was designed by the Leukemia Working Group of the Dutch-Belgian Cooperative Trial Group for Hemato-Oncology (HOVON) and the Swiss Group for Clinical Cancer Research (SAKK) Collaborative Group. Data were collected at the data center of HOVON and the HOVON statisticians conducted the analysis. The study was approved by the ethics committee at each participating institution and was conducted in accordance with the Declaration of Helsinki.
Statistical considerations and response definitions
The definitions of CR, disease-free survival (DFS), and relapse have been described previously.2 Event-free survival (EFS) refers to the interval from randomization to the date of the evaluation of response after the last induction cycle if CR had not been achieved by that time, the date of death, or the date of relapse. Overall survival (OS) was measured from randomization. Early death refers to death within 30 days after randomization. Time to hematopoietic recovery was measured from the first day of chemotherapy to the time when the neutrophil count reached 0.5 and 1.5 × 109/L and the platelet count reached 50 and 100 × 109/L.
Decisions regarding dose escalation to 10 mg/kg, continuation with the initial dose level of 5 mg/kg, or stopping the drug were based on the incidence of DLTs in the bevacizumab arm. A DLT was defined as death within 30 days after the start of cycle 1 and before initiation of start of cycle 2 irrespective of the cause of death. In the previous HOVON/SAKK AML study in older AML patients,2 the incidence of DLT defined in this way was 13%. The latter value was used as a reference estimate in our calculations. A patient was regarded as evaluable for toxicity if he or she had experienced a DLT or was still alive at day 30 after the start of cycle 1. The rules used during part A of the study and the results that determined dose escalation are presented in the supplemental Appendix (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
The primary end points of this study were incidence of DLTs during the initial phase of dose selection (part A of the study) and CR rates during part B of the study. The secondary end points were EFS and OS. Additional secondary end points were the flow cytometric evaluations of minimal residual disease, which will be reported separately.
All analyses were performed according to the intention to treat principle. Hematologic recovery was analyzed actuarially and was compared between the groups by log-rank test. The reported P values are 2-sided and were calculated using the Kruskal-Wallis teset (ie, comparing medians in Tables 1 and 2), the χ2 test (ie, comparing proportions in Tables 1 and 2), the Fisher exact test (ie, comparing proportions in Tables 1 and 2), and Cox log-rank statistics (ie, comparing Kaplan-Meier curves in Tables 1 and 2).
Patient characteristics and outcome parameters
| Variable . | Induction treatment without bevacizumab (n = 85) . | Induction treatment with bevacizumab 10 mg (n = 86) . | . |
|---|---|---|---|
| Age, y | (P = .077, K-W) | ||
| Mean | 67 | 68 | |
| Range | 61-80 | 61-78 | |
| Median | 65 | 67 | |
| Age subgroup, y, n (%) | (P = .042, χ2)* | ||
| 60-64 | 37 (44%) | 22 (26%) | |
| 65-70 | 30 (35%) | 43 (50%) | |
| > 70 | 18 (21%) | 21 (24%) | |
| Male sex, n (%) | 46 (54%) | 48 (56%) | (P = .824, χ2) |
| MDS, n (%) | 11 (13%) | 14 (17%) | (P = .538, χ2) |
| Secondary AML, n (%) | 10 (12%) | 12 (14%) | (P = .670, χ2) |
| WHO performance score, n (%) | (P = .634, FE) | ||
| 0 | 41 (48%) | 43 (50%) | |
| 1 | 37 (44%) | 36 (42%) | |
| 2 | 7 (8%) | 5 (6%) | |
| WBC at diagnosis, n (%) | (P = .555, χ2) | ||
| Mean (range) | 20 (0.9-194) | 27 (0.5-236) | |
| < 20 × 109/L | 65 (76%) | 62 (72%) | |
| > 20-100 × 109/L | 16 (19%) | 16 (19%) | |
| > 100 × 109/L | 4 (5%) | 8 (9%) | |
| Cytogenetic risk, n (%) | (P = .610, FE) | ||
| Favorable | 4 (5%) | 2 (2%) | |
| Intermediate | 43 (51%) | 42 (49%) | |
| Unfavorable | 20 (24%) | 28 (33%) | |
| Very unfavorable | 14 (16%) | 11 (13%) | |
| No cytogenetics available | 4 (5%) | 3 (3%) | |
| Cycle 2 given, n (%) | 63 (74%) | 66 (79%) | (P = .497, χ2) |
| Response after 24 mo, n (%) | |||
| CR | 56 (65%) | 56 (65%) | (P = .916, FE) |
| CR, cycle 1 | 42 (75%) | 41 (73%) | (P = .831, FE) |
| CR, cycle 2 | 14 (25%) | 15 (27%) | |
| Relapse | 32 (38%) | 36 (42%) | (P = .726, FE) |
| Died | 61 (72%) | 61 (71%) | |
| Died in CR | 4 (5%) | 6 (7%) | |
| Variable . | Induction treatment without bevacizumab (n = 85) . | Induction treatment with bevacizumab 10 mg (n = 86) . | . |
|---|---|---|---|
| Age, y | (P = .077, K-W) | ||
| Mean | 67 | 68 | |
| Range | 61-80 | 61-78 | |
| Median | 65 | 67 | |
| Age subgroup, y, n (%) | (P = .042, χ2)* | ||
| 60-64 | 37 (44%) | 22 (26%) | |
| 65-70 | 30 (35%) | 43 (50%) | |
| > 70 | 18 (21%) | 21 (24%) | |
| Male sex, n (%) | 46 (54%) | 48 (56%) | (P = .824, χ2) |
| MDS, n (%) | 11 (13%) | 14 (17%) | (P = .538, χ2) |
| Secondary AML, n (%) | 10 (12%) | 12 (14%) | (P = .670, χ2) |
| WHO performance score, n (%) | (P = .634, FE) | ||
| 0 | 41 (48%) | 43 (50%) | |
| 1 | 37 (44%) | 36 (42%) | |
| 2 | 7 (8%) | 5 (6%) | |
| WBC at diagnosis, n (%) | (P = .555, χ2) | ||
| Mean (range) | 20 (0.9-194) | 27 (0.5-236) | |
| < 20 × 109/L | 65 (76%) | 62 (72%) | |
| > 20-100 × 109/L | 16 (19%) | 16 (19%) | |
| > 100 × 109/L | 4 (5%) | 8 (9%) | |
| Cytogenetic risk, n (%) | (P = .610, FE) | ||
| Favorable | 4 (5%) | 2 (2%) | |
| Intermediate | 43 (51%) | 42 (49%) | |
| Unfavorable | 20 (24%) | 28 (33%) | |
| Very unfavorable | 14 (16%) | 11 (13%) | |
| No cytogenetics available | 4 (5%) | 3 (3%) | |
| Cycle 2 given, n (%) | 63 (74%) | 66 (79%) | (P = .497, χ2) |
| Response after 24 mo, n (%) | |||
| CR | 56 (65%) | 56 (65%) | (P = .916, FE) |
| CR, cycle 1 | 42 (75%) | 41 (73%) | (P = .831, FE) |
| CR, cycle 2 | 14 (25%) | 15 (27%) | |
| Relapse | 32 (38%) | 36 (42%) | (P = .726, FE) |
| Died | 61 (72%) | 61 (71%) | |
| Died in CR | 4 (5%) | 6 (7%) | |
MDS indicates myelodysplastic syndrome; K-W, Kruskal-Wallis; FE, Fisher exact test; WHO, World Health Organization; and WBC, white blood cell.
Multivariate analysis including age group and treatment showed that age group did not influence the end points of this study.
Adverse effects during and after cycles 1 and 2
| Event . | Standard treatment . | Bevacizumab 10 mg cohort . | Significance . | ||
|---|---|---|---|---|---|
| Cycle 1 . | Cycle 2 . | Cycle 1 . | Cycle 2 . | ||
| Maximal-grade side effect, no. of patients | P = .23, exact (both cycles together) | ||||
| Grade 2 | 6 | 7 | 7 | 3 | |
| Grade 3 | 61 | 46 | 61 | 39 | |
| Grade 4 | 16 | 10 | 13 | 22 | |
| Maximal-grade bleedings, no. of patients | P = .12, exact (both cycles together) | ||||
| Grade 2 | 5 | 4 | 7 | 5 | |
| Grade 3 | 3 | 2 | 5 | 4 | |
| Grade 4 | 2 | 0 | 1 | 3 | |
| SAEs, n | 18 | 10 | 26 | 37 | P = .043, χ2 |
| Outcome of SAEs in cycles 1 and 2, no of deaths | 13 (46%) | 22 (35%) | P = .465, χ2 | ||
| Outcome of SAEs in cycles 1 and 2, no of deaths or life-threatening events | 21 (75%) | 38 (60%) | P = .347, χ2 | ||
| Early death, n (%) | 8 (9%) | 6 (7%) | |||
| Nights in hospital, n (median) | |||||
| Cycle 1 | 28 | 29 | P = .448, K-W | ||
| Cycle 2 | 30 | 30 | P = .850, K-W | ||
| Platelet transfusions, n (median) | |||||
| Cycle 1 | 8 | 7 | P = .669, K-W | ||
| Cycle 2 | 9 | 9 | P = .612, K-W | ||
| Event . | Standard treatment . | Bevacizumab 10 mg cohort . | Significance . | ||
|---|---|---|---|---|---|
| Cycle 1 . | Cycle 2 . | Cycle 1 . | Cycle 2 . | ||
| Maximal-grade side effect, no. of patients | P = .23, exact (both cycles together) | ||||
| Grade 2 | 6 | 7 | 7 | 3 | |
| Grade 3 | 61 | 46 | 61 | 39 | |
| Grade 4 | 16 | 10 | 13 | 22 | |
| Maximal-grade bleedings, no. of patients | P = .12, exact (both cycles together) | ||||
| Grade 2 | 5 | 4 | 7 | 5 | |
| Grade 3 | 3 | 2 | 5 | 4 | |
| Grade 4 | 2 | 0 | 1 | 3 | |
| SAEs, n | 18 | 10 | 26 | 37 | P = .043, χ2 |
| Outcome of SAEs in cycles 1 and 2, no of deaths | 13 (46%) | 22 (35%) | P = .465, χ2 | ||
| Outcome of SAEs in cycles 1 and 2, no of deaths or life-threatening events | 21 (75%) | 38 (60%) | P = .347, χ2 | ||
| Early death, n (%) | 8 (9%) | 6 (7%) | |||
| Nights in hospital, n (median) | |||||
| Cycle 1 | 28 | 29 | P = .448, K-W | ||
| Cycle 2 | 30 | 30 | P = .850, K-W | ||
| Platelet transfusions, n (median) | |||||
| Cycle 1 | 8 | 7 | P = .669, K-W | ||
| Cycle 2 | 9 | 9 | P = .612, K-W | ||
K-W indicates Kruskal-Wallis.
All adverse events (AEs) were graded according to National Cancer Institute Common Toxicity-AE Version 3.0. OS, DFS, and EFS curves were estimated by the Kaplan-Meier methodology.
Results
After inclusion of 44 patients in the feasibility part A of the study and according to predefined decision rules, bevacizumab was selected for part B of the study at a dose of 10 mg/kg intravenously at days 1 and 15 after both cycle 1 and cycle 2. Herein the toxicities refer to the total enrolled eligible population (N = 215), whereas demographics and efficacy data are restricted to the patient cohort treated plus or minus the target dose of bevacizumab at 10 mg/kg (n = 171).
Study population
Between March 2007 and August 2009, 219 patients were enrolled in the study. Of the 219 patients, 215 were eligible: 44 in the dose-finding part A of the study (5 mg bevacizumab) and 171 in part B (10 mg bevacizumab). Table 1 lists the characteristics of patients treated in part B of the study. The median age was 66 years. The median age and the number of patients in the higher age categories were higher compared with those in the control arms (P = .042). There were no other significant differences between the 2 groups at baseline.
Treatment, response, and outcome
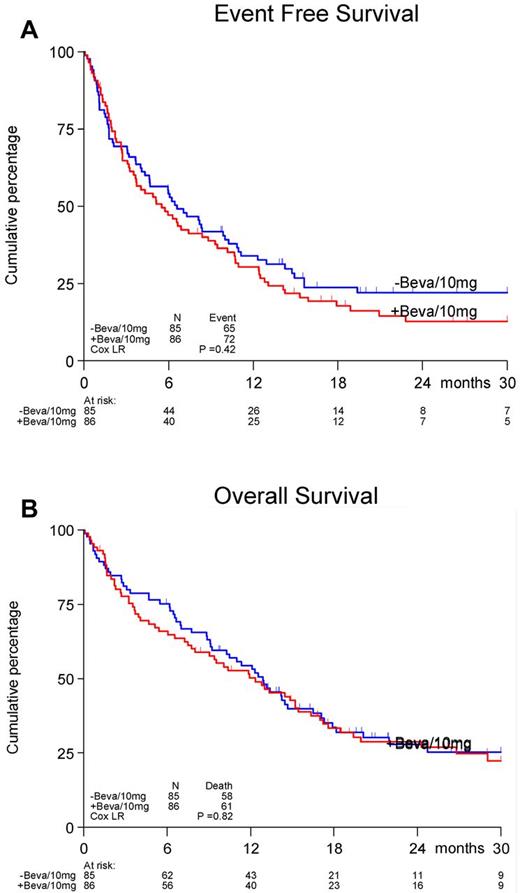
A total of 171 randomized patients received treatment in the first induction cycle, and 129 patients (63 in the control arm and 66 in the bevacizumab arm) received cycle 2. The reasons for not receiving cycle 2 were: toxicity (19%), hypoplasia (5%), death (45%), progression (2%), refusal (7%), and other reasons (21%).The doses of daunorubicin and cytarabine in cycle 1 were delivered according to protocol in 98% of patients and for bevacizumab in 89% of patients. In cycle 2, 90% of patients received the planned cycle of cytarabine and 81% received bevacizumab according to the protocol. No differences between treatment arms were observed. The CR rates in the 2 arms were also not different (65%). Seventy-four percent of patients achieved CR after the first induction cycle with no significant differences between the arms. The upper limit of the 80% confidence interval for the true difference in CR rate was lower than 10% (9.8%), which according to the protocol, indicates evidence for inefficacy. The estimated EFS at 12 months was 33% for the control treatment group without bevacizumab versus 30% for the bevacizumab arm; at 24 months, the EFS was 22% versus 16%, respectively (P = .42, Figure 1A). No differences in OS between the treatment groups were evident (P = .82, Figure 1B). The estimated OS after 24 months was 28% and 29%, respectively. In the control treatment arm, 32 (38%) patients had a relapse, and 4 (5%) died in CR. In the bevacizumab investigational arm, 36 (42%) patients had a relapse and 6 (7%) died in CR.
Effect of addition of bevacizumab to a standard regimen of daunorubicin and cytarabine-arabinoside. Shown are EFS (A) and OS (B) in patients 60 years of age or older with AML.
Effect of addition of bevacizumab to a standard regimen of daunorubicin and cytarabine-arabinoside. Shown are EFS (A) and OS (B) in patients 60 years of age or older with AML.
Toxicities
In supplemental Table 1, the number of AEs in cycles 1 and 2 by diagnosis category, common toxicity criteria (CTC) grade, and arm of randomization are given. The frequencies of grade 3 + 4 CTCs appear generally similar in both arms. A more detailed analysis of events known to be associated with bevacizumab showed a modest increase in hemorrhages in the bevacizumab-treated group (8% vs 13%; nonsignificant) and no difference in thromboembolic complications (6% vs 3%). Table 2 shows that the number of severe AEs (SAEs) was higher in the bevacizumab arm compared with the control arm (63 vs 28, P = .043). However, the percentages of death or life-threatening SAEs were lower in the bevacizumab arm (60% vs 75% of the SAEs). The rates of death after SAEs were higher in the standard treatment arm (46% vs 35% of the SAEs). The causes of death were not different between the 2 arms. No SAE broken down to disease category was statistically significantly more prominent in either of the treatment arms. Therefore, the number of SAEs was higher in the bevacizumab arm, whereas in terms of seriousness and outcome, these appeared to be more favorable than those in the control arm. The early death rate was 9% in the control arm and 7% in the bevacizumab-treated patients.
Hematologic recovery
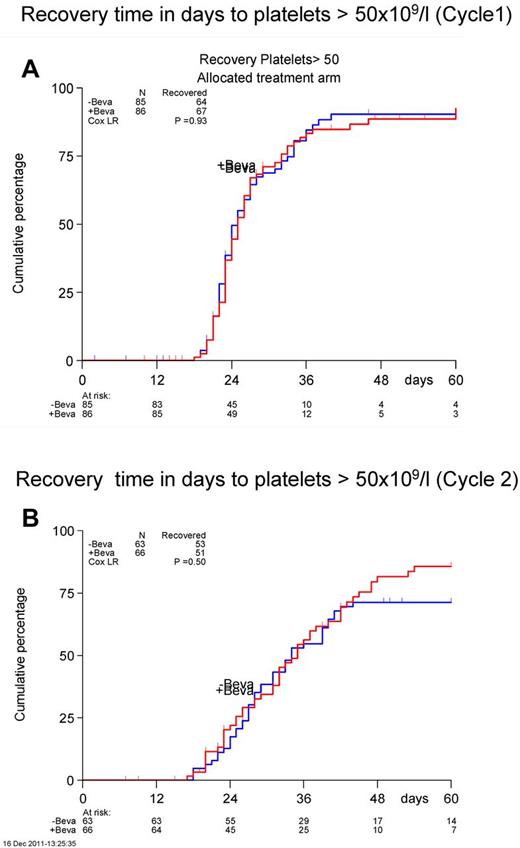
There were no significant differences in time to neutrophil or platelet count recovery neither after cycle 1 or cycle 2 (Figure 2). The median number of platelet transfusions was not different between the 2 arms (cycle 1, 8 vs 7; cycle 2, 9 vs 9). The nights spent in the hospital were also similar between the 2 arms (cycle 1, 28 vs 29; cycle 2, 30 vs 30).
Recovery time in days to achieve a platelet count of > 50 × 109/L. Cycle 1 is shown in panel A and cycle 2 in panel B.
Recovery time in days to achieve a platelet count of > 50 × 109/L. Cycle 1 is shown in panel A and cycle 2 in panel B.
Discussion
This is the first randomized study to evaluate efficacy and toxicity of bevacizumab added to standard first-line intensive remission induction chemotherapy in elderly AML patients. As was shown in a previous HOVON/SAKK cooperative group study in elderly AML patients, a relatively high percentage of CRs (65%) was obtained, which shows the feasibility of delivering high-dose chemotherapy to this age cohort of patients. Unfortunately, the OS is still dismal, mainly because of a high relapse rate. In addition, in this elderly population, patients with a monosomal karyotype appeared to have the worst outcome (supplemental Figure 1). The attempt of HOVON-SAKK to improve on outcome by adding a rationally chosen new drug to the standard treatment (eg, bevacizumab) did not result in a better CR, EFS, or OS. In an attempt to study the biology of angiogenesis in relation to bevacizumab response, we analyzed AML trephine biopsies at diagnosis. The results of this correlative study (A. C. Weidenaar, A. ter Elst, K.v.M., T. G. J. Meeuwsen-de Boer, S. Rosat, M. Bargetzi, C.G., A. Gratwohl, B.L., E.V., G.J.O., E. S. J. M. de Bont, Patterns of bone marrow micro vessel morphology in older patients with AML and high-risk MDS predict treatment outcome following intensive chemotherapy and bevacizumab, manuscript submitted October 2012) revealed different vasculature patterns: a subgroup with low vessel count, a subgroup with “angiogenic sprouting” (ie, a high vessel count with mainly a network of small vessels with thin walls, narrow lumen, and branching), and a subgroup with “vessel hyperplasia” (ie, a high number of vessels with predominantly a large lumen and thin walls), as described previously.15 This correlative study revealed that vasculature morphology and VEGFA mRNA levels did not yield indications for a convincing biomarker for bevacizumab response.
Despite the observations that VEGF plays an important role in the biology of AML and might mediate drug resistance, the results of the present study indicate that therapeutic targeting of VEGF by bevacizumab at the dose given does not exert a measurable positive effect on treatment outcome in elderly AML patients. The safety profile for bevacizumab was comparable to that reported in clinical trials in solid tumors. A greater proportion of SAEs, although not resulting in more deaths, were reported in the bevacizumab-treated patients. No unexpected safety signals were observed. Bleeding complications, thromboembolic events, and hypertension were infrequent and comparable in the 2 treatment arms, as were neutrophil and platelet recoveries and the proportion of patients proceeding to induction cycle 2 after cycle 1.
Bevacizumab has been considered a promising agent as an additive to chemotherapy and had been suggested to offer a therapeutic advantage in patients with epithelial malignancies. However, results in various randomized phase 3 trials have shown variable results and positive effects are mainly expressed in PFS rather than OS. Improvement of survival measured in months has usually been modest, which has currently prompted investigators to reevaluate the exact place of angiogenesis inhibitors in the treatment of certain solid tumor types.16 Because multiple signal transduction pathways are activated in AML, one might speculate that simultaneous inhibition of multiple targets would be more likely to be therapeutically successful, as would the application of specific inhibitors in a selective targeted approach according to the specific cellular and molecular profile of the individual AML patient.
We conclude that preclinical rationale cannot replace empiricism and therefore we should embark on examining a larger number of therapies by performing smaller studies. We should then accept that such trials are nominally underpowered.16 Statistical designs that might be used for rapid evaluation of new therapies in older patients with AML have been proposed.17-19 This should allow for studies of smaller size accepting a somewhat reduced statistical power that can substitute for the large phase 3 studies that have frequently produced negative results. The trial reported herein is an example of such a design based on CR assuming that long-term survival will only be possible in those patients achieving CR. HOVON-SAKK is currently investigating multiple new drugs in a randomized phase 2 multi-arm trial with CR as the primary outcome parameter.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the local and central data managers of the HOVON Data Center for collecting patient data, in particular R. M. Hollestein and C. van Hooije.
This study was supported by the Dutch Cancer Society Queen Wilhelmina Foundation.
The following institutes and investigators of the Dutch-Belgian Cooperative Trial group for Hemato-Oncology (HOVON) and the Swiss Group for Clinical Cancer Research (SAKK) participated in the study: Belgium: Leuven, Gasthuisberg, J. Maertens, GEG Verhoef; Roeselare, Heilig Hart, H. De Muynck; Brussels, St Luc, A. Ferrant; Yvoir, Mont Godinne, A. Bosly, C. Grauw; Antwerp, Hospital Network Antwerp-Stuivenberg-Middelheim, D. A. Breems, R. De Bock; Switzerland: Basel, University Hospital, A. Gratwohl, J. Passweg; Bern, Inselspital, M. Fey, T. Pabst; Geneva, University Hospital (HUG), Y. Chalandon; Aarau, Kantonsspital, M. Bargetzi; Lausanne, Centre Hospitalier Universitaire Vaudois, J. Lambert, Luzern, Kantonsspital, M. Gregor; Zurich, University Hospital, M. Manz, G. Stuessi; The Netherlands: Amersfoort, Meander, S. Wittebol; Amsterdam, Academic Medical Center, B. Biemond; Amsterdam-VUMC, G. Ossenkoppele, A. van de Loosdrecht; Delft, R. De Graaf, R. E. Brouwer; Den Haag, Leyenburg, P. Wijermans; Dordrecht-Albert Hospital Schweitzer, M. Levin; Enschede, Medisch Spectrum Twente, M. Legdeur, W. Smit; Groningen, University Medical Center, G. Huls, E. Vellenga; Heerlen, Atrium, G. Jie; Leeuwarden, MC, M. Hoogendoorn; Leiden, LUMC, C. J. M. Halkes; Maastricht, AZM, H. Schouten, G. Bos; Nieuwegein, Antonius, O. de Weerdt; Rotterdam, Erasmus Medical Center, B. Lowenberg, M. Jongen-Lavrencic, P. Sonneveld, G. de Greef; Utrecht, University Medical Center, J. Kuball; Zwolle, Sophia, R. M. van Marwijk Kooy, L. Verdonck.
Authorship
Contribution: G.J.O., G.S., J.M., Y.v.N., and B.L. created and designed the study; G.J.O., K.v.M., and B.L. and performed the research and analyzed the data; R.M.H. collected the clinical data; and all authors assisted in the analysis and/or interpretation of the data and in writing, critically revising, and approving the final version of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Gert J. Ossenkoppele, MD, PhD, VU University Medical Center, Department of Hematology (2 BR 016), PO Box 7057, 1007 MB Amsterdam, The Netherlands; e-mail: g.ossenkoppele@vumc.nl.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal