Abstract
SOCS3 is a feedback regulator of cytokine signaling that affects T-cell polarization. Human tuberculosis is accompanied by increased SOCS3 expression in T cells, and this may influence susceptibility against Mycobacterium tuberculosis. Because the role of SOCS3 in human T-cell function is not well defined, we characterized cytokine expression and proliferation of human T cells with differential SOCS3 expression in the present study. We established a flow cytometry–based method for SOCS3 protein quantification and detected higher SOCS3 levels induced by M tuberculosis specific T-cell activation and a transient decrease of SOCS3 expression in the presence of mycobacteria-infected macrophages. Notably increased SOCS3 expression was detected in IL-17–expressing T-cell clones and in CD161+ T helper type 17 cells ex vivo. Ectopic SOCS3 expression in primary CD4+ T cells by lentiviral transduction induced increased IL-17 production but diminished proliferation and viability. Recombinant IL-7 inhibited SOCS3 expression and reduced IL-17–expressing T-cell proportions. We concluded that higher SOCS3 expression in human T cells favors T helper type 17 cells. Therefore, increased SOCS3 expression in human tuberculosis may reflect polarization toward IL-17–expressing T cells as well as T-cell exhaustion marked by reduced proliferation.
Introduction
T helper type (TH) 1 cells are crucial for protective immunity against infectious diseases caused by intracellular pathogens, such as Mycobacterium tuberculosis. In this context, exact regulation and limitation of T-cell responses are important and therefore have to be tightly regulated to avoid harmful hyper-reactivity. Important targets for regulation are cytokine receptors, mainly those containing common γ-chains. These receptors signal via the JAK and STAT pathway. Regulation of JAK/STAT signaling is mediated by members of the suppressor of cytokine signaling (SOCS) family.1 The SOCS family is composed of 8 proteins and at least 5 (ie, CISH, SOCS1, SOCS2, SOCS3, and SOCS5) are expressed in T cells.2 SOCS molecules are induced by different stimuli (predominantly cytokines) and act as feedback inhibitors. Inhibition is exerted by different mechanisms, including (1) inhibition of STAT recruitment to cytokine receptors, (2) targeting cytokine receptors and JAK molecules for proteasomal degradation, and (3) direct inhibition of kinase function. Particularly SOCS1 and SOCS3 are crucial for immune functions because modulation of SOCS1 or SOCS3 expression leads to severe immune disorders and embryonic lethality.3
In T cells, the interaction of different SOCS molecules with STAT family members is crucial for proliferation and polarization toward distinct TH cell subtypes.2 Increased SOCS3 expression has been shown to inhibit T-cell proliferation by targeting STAT5 phosphorylation in IL-2 receptor signaling4,5 and CD28-induced IL-2 production.6 Furthermore, SOCS3 has been demonstrated to favor TH2 polarization,7-10 and increased SOCS3 expression promotes TH2-mediated diseases, such as inflammatory arthritis,11 ovalbumin-induced airway hyper-responsiveness,12,13 and liver injuries.14,15 More recent studies demonstrated inhibition of TH17 by SOCS3 in animal models, and this is mediated via regulation of STAT3.16,17 Prerequisites for differentiation of TH17 are controversially discussed and may partly differ between mice and humans.17,18 This situation became even more complex because instability of TH17 cell phenotype, including coexpression of master regulators of ROR-γt (RORC in humans), and T-bet indicated that TH17 are a transient state rather than a stable T-cell population.19-21 Differentiation of TH17 can be subdivided in at least 2 stages: a polarization and an expansion stage. IL-6 is an essential factor for polarization of naive CD4+ T cells toward (pre)TH17, and IL-23 is a crucial for TH17 expansion. Both cytokines, IL-6 and IL-23, exert their function in a STAT3-dependent manner18,22 ; therefore, SOCS3-mediated regulation may affect distinct stages of TH17 generation. CD161 is a marker of naive TH17 precursor as well as mature TH17 cells within the CD4+ T-cell population in humans.23
Recently, we and others identified differential SOCS3 expression in blood and T cells of patients with active tuberculosis.24,25 Besides its crucial role in T-cell polarization, SOCS3 is involved in the exhaustion of T cells, which is an important feature of chronic viral infections.26 In this model, suppression of SOCS3 by IL-7 restored the function of antiviral T cells and led to more effective viral clearance.26
Several studies characterizing the role of SOCS3 on T cells in animal models have been performed, but the function of SOCS3 in human T cells has not been well defined. Consequently, we aim at characterizing the role of SOCS3 in human T cells in the present study. Initially, we established a flow cytometry–based method for SOCS3 protein quantification. This method was used for determination of SOCS3 in different T-cell populations, after in vitro restimulation, and in T-cell clones. Ectopic SOCS3 expression in primary human T cells using a lentiviral-based expression system was then applied to characterize functional changes induced by SOCS3 overexpression. Finally, recombinant IL-7 was used to study the effects of SOCS3 down-regulation on T-cell function and polarization.
Methods
Human subjects
PBMCs were isolated from blood (20 mL) of voluntary healthy donors recruited at the Bernhard-Nocht-Institute for Tropical Medicine and from buffy coats from the Institute for blood transfusion at the University Medical Center Eppendorf in Hamburg. M tuberculosis–infected donors were identified as described previously.27 All donors gave informed consent in accordance with the Declaration of Helsinki, and the local ethics committee approved this study (W-007/09).
SOCS3 expression analyses by flow cytometry
A polyclonal rabbit-α-human SOCS3-antibody (Abcam) was labeled with DyLight 488 or DyLight 649 fluorescent dye (Pierce Chemical) following the manufacturer's instructions. For SOCS3 analysis, T cells or T-cell clones were fixed and permeabilized with Cytofix/Cytoperm solution (BD Biosciences) following the manufacturer's instructions. The cells were then stained intracellularly with different antibodies and labeled αSOCS3 antibody. Antibody specificity was determined by preincubation of 10 μg/mL αSOCS3 antibody with different concentrations of the SOCS3 peptide used to generate the antibody (C-terminal 26 aa, Abcam) or a randomly selected irrelevant peptide for 30 minutes at room termparature. Flow cytometric analyses were generally performed using LSRII flow cytometer (BD Biosciences) and FCS Express Version software (DeNovo). For detailed descriptions of different procedures concerning analysis of T-cell subpopulations, in vitro stimulated T cells, and T-cell clones, see supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
SOCS3 analysis by Western blot
CD4+ T cells (7.5 × 105 cells/mL) were stimulated with αCD3/CD28 (Invitrogen, 4 × 105 beads/mL) for 2 days. A total of 3 × 106 T cells were then lysed in 150-μL sample buffer (50mM Tris-base, 2% SDS, 5% glycerol, bromophenol blue, pH 6.8, supplemented with 100mM DTT). Denaturation of proteins was performed for 15 minutes at 70°C. The soluble proteins were separated using a 15% polyacrylamide gel and subsequently blotted on a nitrocellulose membrane. After blocking for 1 hour at room temperature (PBS, 3% BSA, 0.5% Tween 20) the following primary antibodies were incubated overnight at 4°C: polyclonal antibody rabbit-αSOCS3 (0.5 μg/mL, Abcam) alone or preincubated (30 minutes at room temperature) with the SOCS3 peptide (0.5 μg/mL), the antibody was raised against (C-terminal 26 aa, Abcam), or monoclonal antibody mouse-α-β-actin (0.5 μg/mL, Santa Cruz Biotechnology). Afterward, the following secondary antibodies were incubated for 45 minutes at room temperature: goat-αrabbit-IgG-HRP (Dako North America), rabbit-αmouse-IgG-HRP (Dako North America). Blots were developed for 5 minutes using 1 mL ECL (Pierce Chemical).
Generation of T-cell clones
Purified PBMCs (2 × 107) were stained with mAbs (αCD4 APC-Cy7, αCD45RO PE-Cy-7, both BD and αCD161 FITC, Miltenyi Biotec), and CD4+ CD45ROhighCD161high or CD4+ CD45ROhighCD161low T cells were sorted into 96-well, round-bottom plates (1, 2, 3, 5, or 10 cells/well) using a FACSAria II (BD Biosciences). The plates were precoated with the T-cell stimulating αCD3 antibody (OKT3) before sorting and irradiated heterologous antigen-presenting cells (1 × 105 cells/well) were added. On days 0, 7, 14, and 21, IL-2 (20 U/mL) was added. After 14 days, expanded T-cell clones were counted for each plate. Only T-cell clones from plates with < 33% positive wells were selected to ensure derivation from a single precursor cell.
Coculture of BCG-GFP infected MDMs with PPD-specific T cells
Freshly isolated PBMCs (2 × 106 cells/well) from latently M tuberculosis infected donors were stimulated with purified protein derivative (PPD) of M tuberculosis (2 μg/mL) for 6 days. Concomitantly autologous monocytes were isolated using CD14-labeled magnetic beads (BD Biosciences) and the IMag system (BD Biosciences) following the manufacturer's instructions. The average purity determined by flow cytometry was > 95%. Enriched monocytes (1 × 105/well) were cultured in RPMI 1640 medium (PAA; supplemented with 7.5% FCS, 1% L-glutamine) cells for 6 days to generate monocyte-derived macrophages (MDMs). Afterward, MDMs were infected with GFP-expressing Mycobacterium bovis BCG (MOI 5:1) for 3 hours at 37°C. Then 5 × 105 cells/well of the PPD-stimulated PBMCs were cocultured with BCG-GFP–infected or noninfected MDMs for 3, 8, and 20 hours. Afterward, SOCS3 expression was analyzed by flow cytometry, and relative expression values have been compared between T cells cocultured with BCG-GFP infected and noninfected MDMs.
Ectopic SOCS3 expression by lentiviral gene transduction
For ectopic expression of SOCS3, the open reading frame of human SOCS3 was cloned into the lentiviral LeGO-iG2 vector28 using the restriction sites BamHI and EcoRI (oligos 5′-GCGCGGATCCATGGTCACCCACAGC-AAG-3′ and 5′-GCGCGAATTCTTAAAGC-GGGGCATCGTACT-3′). In this vector, SOCS3 was expressed under the control of a retroviral spleen focus-forming virus promoter concomitantly with eGFP under the control of an internal ribosomal entry side. As a control, vector LeGO-G2 was used, which encodes for eGFP under a spleen focus-forming virus promoter. Lentiviral particles were produced by transient transfection of HEK293T cells as described previously29 using a third-generation lentiviral transduction system.30 Briefly, 5 × 106 HEK293T cells were cotransfected with the SOCS3 expression vector (20 μg), pMDLg/pRRE (10 μg; Addgene; which encodes for lentiviral gag/pol), pRSV-Rev (5 μg; Addgene; which encodes for lentiviral rev), and phCMV-GALV-C4070A (5 μg; which encodes for the gibbon ape leukemia virus (GALV)–envelope protein, kindly provided by C. Stocking, Heinrich-Pette-Institut, Hamburg, Germany). The cotransfection was performed using the calciumphosphate transfection method with 2× HBS (Sigma-Aldrich) and CaCl2 (2.5M). Virus-containing supernatants were harvested twice at 24 and 48 hours after transfection, respectively. The relative virus titer was determined by transduction of HEK293T cells in the presence of Polybrene (8 μg/mL) and flow cytometry analysis of eGFP expression 3 days after infection. Titers of 5 × 106-2 × 107 infectious units/mL were obtained. CD4+ T cells were transduced with lentiviral particles at an MOI of 10 using retronectin (TaKaRa) according to the manufacturer's instructions. In brief, CD4+ T cells were isolated with the IMag system (BD Biosciences) using anti-CD4 antibodies attached to magnetically labeled beads following the manufacturer's instructions. Then 7.5 × 105 cells/mL were prestimulated for 2 days with αCD3/CD28 (see “SOCS3 analysis by Western blot”). The 24-well plates were precoated with 9 μg/cm2 Retronectin (TaKaRa). Plate preloading with viral particles was performed by centrifugation (60 minutes, 1000g, 4°C). The supernatant was discarded, and 2.5 × 105 stimulated T cells in X-Vivo 15 medium (500 μL) were added. On day 1, fresh X-Vivo medium was added.
IL-7 supplementation
For analysis of IL-7–dependent changes in SOCS3 expression and cytokine production, recombinant IL-7 (20 ng/mL; Sigma-Aldrich) was added once on the day of transduction. For cytokine expression analyses in IL-7–treated and non–IL-7–treated samples, ratios (“IL-7–treated”/“non-IL-7–treated” T cells) of IFN-γ, TNF-α, and IL-17-expressing T-cell proportions were determined for samples of individual donors. IL-2 was not added in these experiments.
Proliferation analyses
For analyses of T-cell proliferation in transduced T cells, the cell proliferation dye eFluor670 (eBioscience) was applied following the manufacturer's instructions. Proliferation of T cells was analyzed by flow cytometry calculating the median fluorescence intensities (MFIs) of cell proliferation dye eFluor670 on days 2-7 after transduction. Decreased MFIs correlate with increased proliferation. For cytokine analyses in “fast” and “slow” proliferating T cells, proliferation dye labeled T cells were restimulated on day 5 after transduction with phorbol myristate acetate (PMA; 10 ng/mL) and ionomycin (1 μg/mL) and IFN-γ, TNF-α, and IL-17–expressing T cells were determined by intracellular cytokine staining (as described in “Analyses of cytokine expression by intracellular cytokine staining”). Proliferation dyehigh (“slow” proliferating) and dyelow (“fast” proliferating) T cells were gated and analyzed for the proportions of cytokine expression. Afterward, the ratio (proliferation dyehigh/dyelow) was calculated for each cytokine. Quadruplicates of 4 donors have been included in these experiments.
Analyses of cytokine expression by intracellular cytokine staining
Cytokine expression of transduced T cells was determined after 6 hours in vitro restimulation with PMA (10 ng/mL) and ionomycin (1 μg/mL) in presence of brefeldin A 2 days after transduction. After fixation and permeabilization (as described in “SOCS3 expression by flow cytometry”), the following antibodies were used for staining: αTNF-α AlexaFluor-700 (BD Biosciences), αIFN-γ PE-Cy7 (BD Biosciences), αIL-4 PE (BD Biosciences), and αIL-17 Horizon V450 (BD Biosciences).
Analysis of cytokine concentration in the supernatant
For analysis of cytokine expression in the supernatant, eGFPpositive (SOCS3 and control vector) and eGFPnegative T cells were sorted 2 days after transduction by FACS. Purified T cells (3 × 104/well) were stimulated with PMA/ionomycin (see paragraph: “Analyses of cytokine expression by intracellular cytokine staining”) without the Golgi inhibitor brefeldin A. Supernatants were then harvested 3 days after stimulation, and cytokine analysis was performed with the cytometric beads array (human TH1/TH2/TH17 Kit; BD Biosciences) following the manufacturer's instructions. For data analyses, the FCAP Array Software Version 1.0.2 was used. A factor of 3.5, used for normalization of differential cell numbers between SOCS3-transduced T cells and controls, was calculated of 4 independent experiments. Because of the high numbers of T cells needed for sorting in the cytometric beads array experiments, only 3 experiments were performed.
Statistical analyses
Statistical analyses of comparisons between 2 independent groups have been performed using the Student t test (for Gaussian distributions) or the Mann-Whitney U test (for non-Gaussian distributions) according to Kolmogorov-Smirnow normality testing (SigmaStat Software Version 3.5). The paired t test was applied to assess differences of SOCS3 expression induced by in vitro stimulation with PPD and αCD3/CD28, in TH17 (CD161high), and TH2 (CRTH2high) precursor cells, for cytokine analysis after SOCS3 lentiviral transduction, and stimulations in presence or absence of IL-7. The Mann-Whitney U test was applied to assess SOCS3 expression differences in T-cell clones. Statistical analyses were accomplished using the GraphPad Prism Software Version 5.0 or the SigmaStat Software Version 3.5. Nominal 2-sided P values given were considered significant if P < .05.
Results
Quantitative assessment of SOCS3 protein expression by flow cytometry
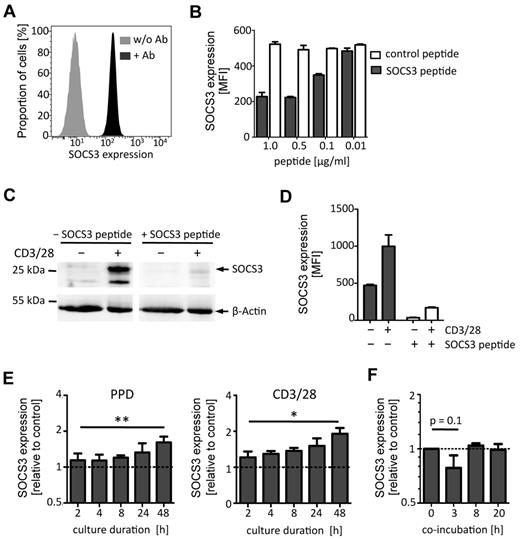
Previous studies determined SOCS3 expression using real-time PCR for RNA quantification or Western blots for semiquantitative protein analysis. To precisely quantify SOCS3 protein expression in a cell-specific manner, we established antibody-based flow cytometric analysis for quantification of SOCS3. A polyclonal rabbit-α-human SOCS3 antibody was fluorescently labeled, and MFIs of different antibody concentrations were determined intracellularly. Results of a representative experiment indicating SOCS3 MFIs of PBMCs are shown as Figure 1A. Depending on the labeling efficiency, an antibody concentration of ∼ 10 μg/mL was sufficient to detect SOCS3, and adjusted antibody concentrations were therefore used throughout the experiments. The capability of this flow cytometric assay to specifically quantify SOCS3 protein expression based on MFI was determined by applying antibodies preincubated with increasing concentrations of the SOCS3 peptide epitope. The SOCS3 peptide epitope markedly reduced the MFI, and 0.5 μg/mL of the peptide was sufficient to block SOCS3 binding at most (Figure 1B). Higher concentrations had no additional effect, and a randomly chosen control peptide did not affect binding of the antibody at any concentration (Figure 1B). A 50% inhibition level was detected for a peptide concentration of 0.1 μg/mL. Because SDs of MFI between replicates in the same experiment are moderate (Figure 1B), we conclude that MFI measure is a highly accurate method for quantification of SOCS3 protein expression.
SOCS3 protein expression analysis by flow cytometry. SOCS3 expression of PBMCs and activated T cells was determined by staining with a polyclonal SOCS3-specific antibody (αSOCS3 Ab). (A) Flow cytometric histogram showing SOCS3 expression (MFI; x-axis) of PBMCs with SOCS3-specific antibodies (10 μg/mL; black curve) or without (gray curve). (B) SOCS3 staining of PBMCs after preincubation of antibodies with different concentrations of SOCS3 peptide (gray bars) or control peptide (open bars). MFIs for SOCS3 are shown on the y-axis. Bars represent mean ± SD of 2 replicates. One representative experiment of 3 is shown. (C-D) SOCS3 expression analysis of CD4+ T cells after in vitro culture with αCD3/CD28 or without for 2 days is shown. SOCS3 Ab with or without SOCS3 peptide preincubation was performed. (C) Western blot analysis indicates SOCS3 protein expression. β-Actin was used as a control. (D) SOCS3 expression analysis by flow cytometry. SOCS3 expression is indicated with (open bars) or without SOCS3 peptide (black filled bars). (E) SOCS3 expression of in vitro activated T cells at different time points is shown as relative expression of CD40Lhigh (activated) cells compared with CD40Llow (nonactivated) cells (dotted lines). Stimulations with PPD of M tuberculosis or αCD3/CD28 are depicted. Bars represent mean ± SD of 2 replicates; n = 4. *P < .05 (paired t test). **P < .01 (paired t test). (F) SOCS3 expression in PPD-specific T cells, cocultured with BCG-GFP–infected autologous MDMs at different time points. The relative expression of T cells, cocultured with infected MDMs to T cells, cocultured with noninfected MDMs is shown (dotted line). Bars represent mean ± SD of 2 replicates (n = 3).
SOCS3 protein expression analysis by flow cytometry. SOCS3 expression of PBMCs and activated T cells was determined by staining with a polyclonal SOCS3-specific antibody (αSOCS3 Ab). (A) Flow cytometric histogram showing SOCS3 expression (MFI; x-axis) of PBMCs with SOCS3-specific antibodies (10 μg/mL; black curve) or without (gray curve). (B) SOCS3 staining of PBMCs after preincubation of antibodies with different concentrations of SOCS3 peptide (gray bars) or control peptide (open bars). MFIs for SOCS3 are shown on the y-axis. Bars represent mean ± SD of 2 replicates. One representative experiment of 3 is shown. (C-D) SOCS3 expression analysis of CD4+ T cells after in vitro culture with αCD3/CD28 or without for 2 days is shown. SOCS3 Ab with or without SOCS3 peptide preincubation was performed. (C) Western blot analysis indicates SOCS3 protein expression. β-Actin was used as a control. (D) SOCS3 expression analysis by flow cytometry. SOCS3 expression is indicated with (open bars) or without SOCS3 peptide (black filled bars). (E) SOCS3 expression of in vitro activated T cells at different time points is shown as relative expression of CD40Lhigh (activated) cells compared with CD40Llow (nonactivated) cells (dotted lines). Stimulations with PPD of M tuberculosis or αCD3/CD28 are depicted. Bars represent mean ± SD of 2 replicates; n = 4. *P < .05 (paired t test). **P < .01 (paired t test). (F) SOCS3 expression in PPD-specific T cells, cocultured with BCG-GFP–infected autologous MDMs at different time points. The relative expression of T cells, cocultured with infected MDMs to T cells, cocultured with noninfected MDMs is shown (dotted line). Bars represent mean ± SD of 2 replicates (n = 3).
Protein analysis using Western blot detected a band at ∼ 25 kDa in accordance to the predicted molecular weight of SOCS3 and a second, slightly smaller, band matched to a described SOCS3 splice variant (Figure 1C left panel second lane).31 Western blot analyses hardly detected SOCS3 in primary human T cells ex vivo (Figure 1C first lane), whereas markedly increased SOCS3 expression was detected after in vitro T-cell activation (Figure 1C second lane). Again, antibody preincubation with the SOCS3 peptide epitope largely diminished intensities of assumed SOCS3 bands but not β-actin used as a control (Figure 1C fourth lane). Notably, a very moderate decrease was detected in the nonactivated T cells (Figure 1C third lane) compared with the nonpeptide control (Figure 1C first lane). Concomitant flow cytometric analysis proved the marked increase of SOCS3 MFI after T-cell activation and a decrease of SOCS3 MFI by the blocking peptide (Figure 1D). Notably, MFI of nonstimulated T cells, although ∼ 2-fold lower (compared with the stimulated sample), decreased markedly (∼ 14-fold) when SOCS3 peptide was added. These results prove SOCS3 antibody specificity and usability in flow cytometric assays for determination of protein concentrations by MFI. In addition, these results demonstrated the higher sensitivity and accuracy of the flow cytometry–based assay compared with semiquantitative Western blot.
SOCS3 is regulated in human M tuberculosis–specific T cells
Previous studies indicated a role of SOCS3 in human tuberculosis.24,25 Therefore, we characterized SOCS3 expression in activated T cells (CD40Lhigh) after in vitro stimulation with M tuberculosis antigen (PPD). PPD induced moderately increased SOCS3 expression in activated T cells with a maximum of 2.5-fold after 48 hours compared with CD40Llow (nonactivated) T cells (Figure 1E). The PPD-specific SOCS3 induction was comparable with the polyclonal T-cell activator αCD3/CD28, which induced up to 2-fold increased SOCS3 expression in CD40Lhigh T cells (Figure 1E). Increased SOCS3 expression levels of in vitro activated T cells were stable for at least a week (data not shown).
To characterize the direct effect of mycobacterial infection on SOCS3, we established an in vitro assay based on T-cell interaction with M bovis BCG-GFP (BCG) infected monocyte-derived macrophages (MDMs). T cells specifically stimulated with mycobacterial proteins were coincubated with autologous MDMs with or without BCG infection. Coincubation with infected MDMs induced a tendency of transient down-regulation of SOCS3 in T cells (P = .1) during the first 3 hours (Figure 1F). We conclude that T-cell activation led to increased expression of SOCS3 and that transient down-regulation of SOCS3 in T cells occurred early during interaction with BCG infected MDMs.
Higher SOCS3 expression in IL-17 T-cell clones and TH17 precursor cells
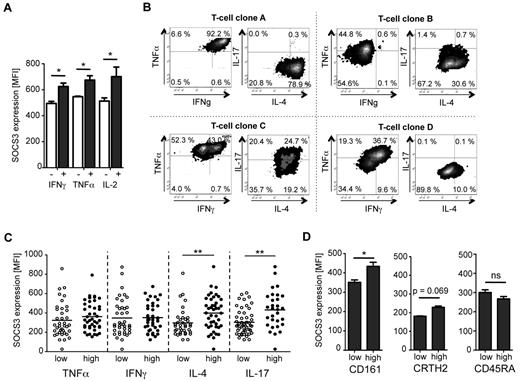
SOCS3 has been described to polarize T-cell immunity, leading to a different cytokine expression pattern. Therefore, we performed concomitant analyses of SOCS3 and cytokine expression after in vitro T-cell stimulation. αCD3/CD28 stimulation induced similar SOCS3 expression levels in IFN-γ, TNF-α, and IL-2–producing T cells (Figure 2A). Hardly any IL-17 and IL-4 was induced after short-term in vitro stimulation. Consequently, we generated T-cell clones based on a single-cell sorting approach using irradiated feeder cells and CD3 cross-linking (for details see “Methods”). This method induced TH1, TH2, and TH17 cells without application of polarizing culture conditions. T-cell clones were then characterized for cytokine expression pattern (ie, TNF-α, IFN-γ, IL-17, IL-4). Representative examples of T-cell clones are shown as Figure 2B. T-cell clones were then classified according to high or low expression of TNF-α, IFN-γ, IL-4, and IL-17, respectively. Similar SOCS3 expression was detected in T-cell clones expressing high or low levels of IFN-γ or TNF-α (Figure 2C). In contrast, significantly higher SOCS3 expression was detected in IL-4high and IL-17high T-cell clones (Figure 2C). Because inhibition of TH17 polarization by SOCS3 has been described before,16,32 we additionally analyzed the ex vivo expression of SOCS3 in CD161high T helper cells (the TH17 originating population in humans33 ). We detected higher SOCS3 expression levels in CD161high T cells compared with CD161low T cells (P = .02, Figure 2D left graph) and a tendency of higher SOCS3 expression in described CRTH2high TH2 cells34 (P = .067, Figure 2D middle graph), whereas no difference was detected between control T-cell populations discriminated by CD45RA expression (Figure 2D right graph). We conclude that SOCS3 expression is increased in IL-17high T-cell clones as well as in primary CD161high polarized TH17 cells.
SOCS3 expression in cytokine-expressing T cells, T-cell clones, and TH17 precursor cells. (A) Analysis of median SOCS3 expression in cytokine-positive (dark gray) and cytokine-negative (open) CD4+ T cells stimulated with αCD3/CD28 for 36 hours. Bars represent median and SD of 2 replicates. One representative experiment of 3 is shown. *P < .05 (paired t test). (B) Cytokine expression profiles of 4 representative T-cell clones. T-cell clones were restimulated with PMA/ionomycin on day 14 after sorting, and expression of TNF-α, IFN-γ, IL-4, and IL-17 was determined by intracellular cytokine staining. (C) SOCS3 expression analyses of T-cell clones with differential cytokine expression. Each circle represents a T-cell clone with high (●) or low (○) expression for the indicated cytokine. n = 80. **P < .01 (Mann-Whitney U test). (D) Ex vivo analysis of SOCS3 expression in TH17 (CD161high) and TH2 (CRTH2high) precursor cells by flow cytometry. SOCS3 expression was determined in CD4+ T-cell populations that show high or low expression for the respective lineage markers. As a control, SOCS3 expression was also analyzed in CD45RAhigh and CD45RAlow CD4+ T cells. One representative experiment of 6 is shown for CD161 and CRTH2 and 1 of 3 for CD45RA. Bars represent mean ± SD of 2 replicates. ns indicates not significant. *P < .05 (paired t test).
SOCS3 expression in cytokine-expressing T cells, T-cell clones, and TH17 precursor cells. (A) Analysis of median SOCS3 expression in cytokine-positive (dark gray) and cytokine-negative (open) CD4+ T cells stimulated with αCD3/CD28 for 36 hours. Bars represent median and SD of 2 replicates. One representative experiment of 3 is shown. *P < .05 (paired t test). (B) Cytokine expression profiles of 4 representative T-cell clones. T-cell clones were restimulated with PMA/ionomycin on day 14 after sorting, and expression of TNF-α, IFN-γ, IL-4, and IL-17 was determined by intracellular cytokine staining. (C) SOCS3 expression analyses of T-cell clones with differential cytokine expression. Each circle represents a T-cell clone with high (●) or low (○) expression for the indicated cytokine. n = 80. **P < .01 (Mann-Whitney U test). (D) Ex vivo analysis of SOCS3 expression in TH17 (CD161high) and TH2 (CRTH2high) precursor cells by flow cytometry. SOCS3 expression was determined in CD4+ T-cell populations that show high or low expression for the respective lineage markers. As a control, SOCS3 expression was also analyzed in CD45RAhigh and CD45RAlow CD4+ T cells. One representative experiment of 6 is shown for CD161 and CRTH2 and 1 of 3 for CD45RA. Bars represent mean ± SD of 2 replicates. ns indicates not significant. *P < .05 (paired t test).
Ectopic SOCS3 expression in CD4+ T cells inhibits proliferation
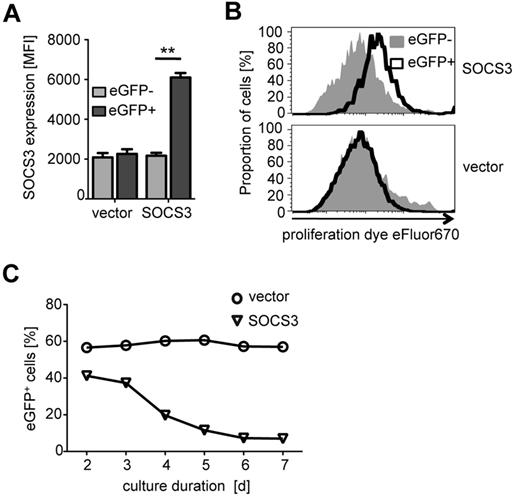
To investigate functional effects of differential SOCS3 expression, we used a lentiviral transduction system for ectopic SOCS3 expression in activated primary CD4+ T cells. Because T-cell receptor specific activation was essential for transduction, we were not able to modulate SOCS3 expression in nonpolarized naive T cells (data not shown). Ectopic SOCS3 was indicated by concomitant eGFP expression. SOCS3 expression analysis by flow cytometry revealed significantly increased expression after lentiviral transduction (P = .008; Figure 3A). The transduction efficiency varied between 50% and 90% for the control vector and 30% and 60% for the SOCS3 vector in different experiments (data not shown). Proliferation analyses revealed a marked reduction of cell divisions in CD4+ T cells with ectopic SOCS3 expression (Figure 3B top histogram) compared with vector control–transduced T cells (Figure 3B bottom histogram). This was accompanied by decreased proportions of SOCS3-transduced T cells during in vitro culture (Figure 3C). We did not detect increased expression of apoptosis- or necrosis-associated markers on SOCS3-transduced T cells (data not shown).
Ectopic SOCS3 expression by lentiviral transduction and effects on proliferation of CD4+ T cells. CD4+ T cells transduced with a lentiviral SOCS3 expression vector coexpressing eGFP, or a control vector expressing eGFP only, after prestimulation with αCD3/CD28 are shown. (A) SOCS3 protein expression analyzed 2 days after transduction for SOCS3 and control vector–transduced CD4+ T cells is shown. EGFPhigh T cells represent lentivirally transduced cells (black bars), and eGFPlow T cells represent nontransduced cells in the same well (gray bars). Bars represent mean ± SD of 2 replicates. One representative experiment of 5 is shown. **P .01 (paired t test). (B) Proliferation analyses of SOCS3- (top graph) and control vector–transduced (bottom graph) T cells on day 6 after transduction using cell proliferation dye eFluor670. Black open curves indicate EGFPhigh cells; and gray curves, eGFPlow cells. Absolute cell numbers (normalized to the maximal cell count) are shown. One representative experiment of 5 is depicted. (C) Proportions of eGFP+ stimulated T cells in vitro (days 2-7) are shown for SOCS3 (triangles) and control vector (circles). One representative experiment of 3 is shown.
Ectopic SOCS3 expression by lentiviral transduction and effects on proliferation of CD4+ T cells. CD4+ T cells transduced with a lentiviral SOCS3 expression vector coexpressing eGFP, or a control vector expressing eGFP only, after prestimulation with αCD3/CD28 are shown. (A) SOCS3 protein expression analyzed 2 days after transduction for SOCS3 and control vector–transduced CD4+ T cells is shown. EGFPhigh T cells represent lentivirally transduced cells (black bars), and eGFPlow T cells represent nontransduced cells in the same well (gray bars). Bars represent mean ± SD of 2 replicates. One representative experiment of 5 is shown. **P .01 (paired t test). (B) Proliferation analyses of SOCS3- (top graph) and control vector–transduced (bottom graph) T cells on day 6 after transduction using cell proliferation dye eFluor670. Black open curves indicate EGFPhigh cells; and gray curves, eGFPlow cells. Absolute cell numbers (normalized to the maximal cell count) are shown. One representative experiment of 5 is depicted. (C) Proportions of eGFP+ stimulated T cells in vitro (days 2-7) are shown for SOCS3 (triangles) and control vector (circles). One representative experiment of 3 is shown.
Ectopic SOCS3 expression in CD4+ T cells promotes IL-17 production
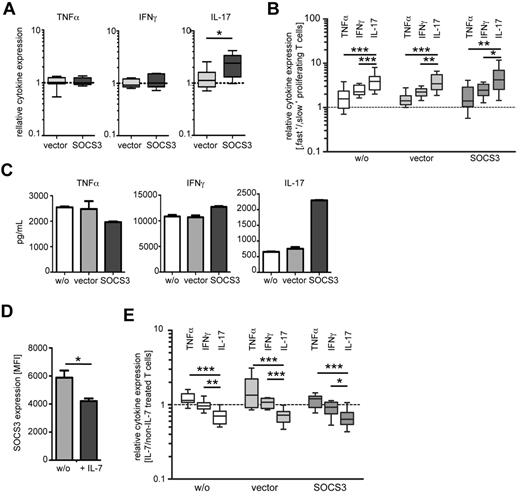
Next, we analyzed cytokine expression of SOCS3-transduced T cells after in vitro restimulation. Polyclonal T-cell activation revealed similar proportions of TNF-α and IFN-γ in T cells with ectopic SOCS3 expression and vector control–transduced T cells (Figure 4A left and middle graphs). Notably, we detected increased proportions of IL-17-producing CD4+ T cells with ectopic SOCS3 expression (P = .015; Figure 4A right graph). IL-4 was not detectable after restimulation. Because differences in cytokine expression induced by SOCS3 can be the result of differential proliferation of distinct TH-cell subpopulations, we next compared cytokine expression of “slow” and “fast” proliferating T cells. Concomitant analyses for cell proliferation and cytokine-expression detected cytokine-positive T cells predominantly in the “fast” proliferating population (Figure 4B). The median ratios (“fast”/“slow” proliferating cells) were between 2/1 and 3/1 for TNF-α and IFN-γ. Notably, the ratios were significantly higher (> 3/1) for IL-17–expressing T cells. Comparable results were gained for nontransduced, vector-transduced, and SOCS3-transduced T cells (Figure 4B). This demonstrated that TH17 cells are even faster proliferating than TH1 cells and does not support the notion that increased IL-17 expression induced by SOCS3 is the result of reduced proliferation.
The influence of modulated SOCS3 expression on cytokine expression of CD4+ T cells. (A) Intracellular cytokine expression of in vitro restimulated CD4+ T cells (PMA/ionomycin) 2 days after ectopic expression of SOCS3 (black) or control vector (gray) by lentiviral transduction. Relative cytokine expression values compared with cells without transduction (dotted line) are shown as box plots. Box plots and error bars represent 5, 25, 50, 75, and 95 percentiles; n = 10. *P < .05 (paired t test). (B) Cytokine expression in “fast” and “slow” proliferating T cells on day 5 after transduction is shown for SOCS3 (black boxes), control vector (gray boxes), and nontransduced (open boxes) T cells, restimulated with PMA/ionomycin. We calculated cytokine expression ratios of “fast” versus “slow” proliferating T cells. Equal expression between “fast” and “slow” proliferating T cells is indicated as a dotted line. Box plots and error bars represent 5, 25, 50, 75, and 95 percentiles; n = 5. *P < .05 (Student t test). **P < .01 (Student t test). ***P < .001 (Student t test). (C) Cytokine concentration determined in the supernatant of SOCS3 (black bars), control vector (gray bars), and nontransduced (open bars) T cells. Before in vitro restimulation with PMA/ionomycin (day 3), cells were enriched according to eGFP expression. Cytokine concentrations normalized to cell numbers of nontransduced cells are shown. Bars represent mean ± SD of 2 replicates. One representative experiment of 3 is shown. (D) SOCS3 expression of CD4+ T cells after 5 days in vitro stimulation with αCD3/CD28 in the presence or absence of recombinant IL-7. Bars represent mean ± SD of 2 replicates. One representative experiment of 4 is shown. *P < .05 (paired t test). (E) Cytokine expression of IL-7–treated cells. IL-7 was added on the day of transduction, and T cells were then restimulated with PMA/ionomycin on 3 days after transduction. Ratios of TNF-α, IFN-γ, and IL-17-expressing T-cell proportions (IL-7–treated/non-IL-7–treated) are shown on the y-axis. The dotted line indicates equal proportions of cytokine-expressing T cells between treatments. Box plots and error bars represent 5, 25, 50, 75, and 95 percentiles; n = 5. *P < .05 (Student t test). **P < .01 (Student t test). ***P < .001 (Student t test).
The influence of modulated SOCS3 expression on cytokine expression of CD4+ T cells. (A) Intracellular cytokine expression of in vitro restimulated CD4+ T cells (PMA/ionomycin) 2 days after ectopic expression of SOCS3 (black) or control vector (gray) by lentiviral transduction. Relative cytokine expression values compared with cells without transduction (dotted line) are shown as box plots. Box plots and error bars represent 5, 25, 50, 75, and 95 percentiles; n = 10. *P < .05 (paired t test). (B) Cytokine expression in “fast” and “slow” proliferating T cells on day 5 after transduction is shown for SOCS3 (black boxes), control vector (gray boxes), and nontransduced (open boxes) T cells, restimulated with PMA/ionomycin. We calculated cytokine expression ratios of “fast” versus “slow” proliferating T cells. Equal expression between “fast” and “slow” proliferating T cells is indicated as a dotted line. Box plots and error bars represent 5, 25, 50, 75, and 95 percentiles; n = 5. *P < .05 (Student t test). **P < .01 (Student t test). ***P < .001 (Student t test). (C) Cytokine concentration determined in the supernatant of SOCS3 (black bars), control vector (gray bars), and nontransduced (open bars) T cells. Before in vitro restimulation with PMA/ionomycin (day 3), cells were enriched according to eGFP expression. Cytokine concentrations normalized to cell numbers of nontransduced cells are shown. Bars represent mean ± SD of 2 replicates. One representative experiment of 3 is shown. (D) SOCS3 expression of CD4+ T cells after 5 days in vitro stimulation with αCD3/CD28 in the presence or absence of recombinant IL-7. Bars represent mean ± SD of 2 replicates. One representative experiment of 4 is shown. *P < .05 (paired t test). (E) Cytokine expression of IL-7–treated cells. IL-7 was added on the day of transduction, and T cells were then restimulated with PMA/ionomycin on 3 days after transduction. Ratios of TNF-α, IFN-γ, and IL-17-expressing T-cell proportions (IL-7–treated/non-IL-7–treated) are shown on the y-axis. The dotted line indicates equal proportions of cytokine-expressing T cells between treatments. Box plots and error bars represent 5, 25, 50, 75, and 95 percentiles; n = 5. *P < .05 (Student t test). **P < .01 (Student t test). ***P < .001 (Student t test).
Next, we determined cytokines in the supernatants of transduced T cells. For these experiments, eGFP+ (transduced) T cells were enriched by FACS before restimulation. We detected comparable IFN-γ and TNF-α levels between T cells with ectopic SOCS3 expression and controls (values were normalized for differential cell counts; for details see “Methods”; Figure 4C). Notably, as for intracellular cytokines, a marked increase of IL-17 was detected in the supernatant of T cells with ectopic SOCS3 expression (Figure 4C). We conclude that ectopic SOCS3 expression reduced proliferation of activated T cells and induced increased proportions of IL-17–producing T cells.
IL-7 reduces endogenous SOCS3 expression and leads to decreased Th17 proportions
The effects of SOCS3 down-regulation were addressed using described small hairpin (sh)RNAs for SOCS3 in a lentiviral vector (for details see supplemental Methods). We analyzed 5 commercially available SOCS3-specific shRNAs for reduction of SOCS3 expression in HEK293T cells using flow cytometry (supplemental Figure 1A) and Western blot (supplemental Figure 1B). None of these shRNAs down-regulated SOCS3 protein expression consistently, whereas eGFP-specific control shRNA reduced eGFP expression (supplemental Figure 1C). Similar results were gained for CD4+ T cells (data not shown). Therefore, we were not able to analyze the influence of SOCS3 down-regulation using shRNAs.
Recently, it has been shown that IL-7 inhibits SOCS3 expression in exhausted T cells from mice with chronic viral infections.26 Therefore, we determined the effect of IL-7 on SOCS3 expression of human CD4+ T cells and accompanied functional differences. Treatment with recombinant IL-7 before or after lentiviral transduction of T cells did not affect SOCS3 transduction efficiency (data not shown) but led to reduced endogenous expression of SOCS3 (∼ 20%; P = .045; Figure 4D). Analyses of cytokine expressing T-cell proportions (ratios of “IL-7–treated”/“non-IL-7–treated” samples) revealed slightly increased proportions of TNF-α-expressing T cells on IL-7 treatment (Figure 4E), whereas IFN-γ-expressing T cells were similar between IL-7–treated and nontreated T cells (Figure 4E). Notably, IL-17-expressing T-cell proportions were significantly reduced compared with IFN-γ and TNF-α-producing T cells when IL-7 was added to culture (Figure 4E). These results were concordant between SOCS3-transduced T cells and controls. Therefore, IL-7–treated T cells with reduced SOCS3 expression comprised decreased TH17 proportions. These results strengthen the hypothesis that SOCS3 promotes TH17 cells.
Discussion
In the present study, we show that SOCS3 influences human T-cell functions and polarization. Our data suggest that increased SOCS3 expression promotes (1) IL-4–producing T-cell clones, (2) IL-17–producing primary T cells, T-cell clones, and TH17 precursor cells, and (3) decreased proliferation of T cells in vitro. IL-7–induced inhibition of SOCS3 expression leads to reduced proportions of IL-17–producing TH17 cells.
SOCS3 has been shown to influence polarization of CD4+ T cells. In this context, an enhancing effect of SOCS3 on the generation of IL-4–producing TH2 cells has been demonstrated.7-10 This SOCS3-mediated TH2 promoting effect is the result of inhibition of TH1 by blocking STAT4 phosphorylation.7 In accordance, we detected higher SOCS3 expression in IL-4–expressing T-cell clones as well as a tendency of higher SOCS3 expression in CRTH2high-expressing TH2 cells34 in the present study. Because short-term in vitro stimulation hardly induced any IL-4–producing TH2 cells, we were not able to determine TH2 proportions in response to ectopic SOCS3 expression by lentiviral transduction of primary T cells.
More recent studies did not observe an influence of SOCS3 on TH2 polarization, but rather an inhibitory effect of SOCS3 on TH17 cells.16,35 In contrast, the results from the present study did not suggest an inhibitory effect of SOCS3 on TH17. We identified increased SOCS3 expression in T cell clones with predominant TH2 and TH17 cytokine expression pattern. In addition, ex vivo analyzed TH17 (CD161high) cells exhibited increased SOCS3 expression and TH2 (CRTH2high) cells showed a tendency of higher SOCS3 expression. In these experiments SOCS3 expression differences may be underestimated because TH17 cells are only a fraction of CD161high T cells.23 Increased proportions of TH17 cell were also found induced by ectopic SOCS3 expression. Altogether, these results suggested a promoting role for SOCS3 in human TH17 development.
The molecular mechanisms how increased SOCS3 expression favors human TH17 cells remain elusive. The immune polarizing effect of SOCS3 is mainly assigned to its STAT inhibitory functions. In this context, SOCS3 blocks phosphorylation of STAT4, the main inducer of TH1,7,36 and phosphorylation of STAT3, the critical inducer of TH17.16,37 A possible explanation for our contrary findings concerning TH17 is that SOCS3 inhibits polarization of noncommitted naive T cells (TH0) toward TH17, whereas differentiated TH17 or preTH17 cells are not (or less) affected by SOCS3-mediated inhibition of STAT3 phosphorylation (eg, by differential effects of SOCS3 on distinct cytokine receptor–mediated STAT3 phosphorylation38 ). This thesis could not be examined in the present study because of assay restrictions and limitations of the human model. Among others, analysis of CD161 cannot distinguish between T-cell differentiation stages because CD161 is expressed on TH17 as well as on naive TH17 precursors. Furthermore, activation of T cells before lentiviral transduction is a prerequisite for the method; therefore, we were not able to analyze the influence of SOCS3 on nonpolarized T cells in this assay. Nevertheless, because short-term in vitro stimulation with low concentrations of T-cell stimulating beads activates predominantly effector and memory cells (M.J., unpublished data, March 2008), it is justified to conclude that SOCS3 modulation by lentiviral transduction affected precommitted TH17 cells and not naive TH17 precursors in our assay. Experiments that address the point whether distinct cytokine receptors (eg, IL-23 or IL-6 receptors) are differentially affected by SOCS3 are difficult to perform in our experimental setting because SOCS3 simultaneously inhibits proliferation (Figure 3B) and SOCS3-transduced T cells disappear in culture within a few days (Figure 3C).
Besides STAT-dependent regulation of T-cell differentiation by SOCS3, other mechanisms are also possible. Quintana et al described very recently that down-regulation of IL-2 expression by the transcription factor Aiolos promoted TH17 differentiation.39 SOCS3 has also been shown to inhibit IL-2 by directly blocking activation of CD28.6 We conclude that SOCS3 may affect TH17 polarization in different ways. Ongoing studies aim at deciphering this complex picture to clarify the role of SOCS3 in T-cell polarization.
Recombinant IL-7 has recently been described to directly inhibit SOCS3 expression of CD8+ T cells in animal models of chronic viral infections.26 In the present study, we demonstrated that IL-7 inhibited SOCS3 also in human CD4+ T cells. Because we were not able to down-regulate SOCS3 using shRNAs, we applied recombinant IL-7 to suppress SOCS3. Here we observed a promoting effect of IL-7 predominantly on TNF-α-producing T cells, which were more frequent in the presence of IL-7, whereas IFN-γ–expressing T cells were found at similar frequencies. In contrast, IL-17–producing T cells decreased in the presence of IL-7, and this has been shown by others, too.40 We cannot exclude that IL-7 has additional effects on T-cell functions besides SOCS3 suppression; therefore, exclusive inhibition of SOCS3 would be important to verify these results. Despite the limitations of this assay, we conclude that the IL-17–suppressive effect of IL-7 supported our hypothesis for a TH17 promoting role of SOCS3.
In the present study, we showed that ectopic SOCS3 expression in CD4+ T cells strongly inhibited proliferation. This is in accordance with previous studies that showed reduced proliferation of T cells caused by SOCS3-mediated blocking of CD28 coreceptor activation.4,6,41,42 Defective proliferation is a feature of T-cell exhaustion that has been attributed to T cells in chronic viral diseases, and SOCS3 plays a central role in these processes.26 It is tempting to speculate that T-cell exhaustion also plays a role in human tuberculosis where SOCS3 expression is increased during active disease.24,25 Previously, we identified exhausted CD8+ T cells in children with severe forms of tuberculosis.43
Besides infectious diseases, SOCS3 plays an important role in autoimmunity44 and tumors.45 Consequently, SOCS3 has become a target for interventive therapy.2,46 Against this background and controversial results in animal models, we would strongly suggest to place the role of SOCS3 in T-cell polarization and exhaustion on today's agenda. In this context, we consider the present study an important step to elucidate the function of SOCS3 in human T-cell polarization.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank B. Kretschmer for comments on the manuscript and M. Milsom for technical help, particularly the protocol for cloning of shRNAs from pLKO.1 into LeGO-G.
This work is part of the PhD thesis of K.K.
This work was supported by the Deutsche Forschungsgemeinschaft project, Immune polarization in childhood TB (JA 1479/3-1; S.S. and M.J.). U.M. was supported by the BMBF within the iGene consortium.
Authorship
Contribution: K.K. performed experiments, analyzed the data, and wrote the manuscript; K.H. contributed to the establishment and generation of T-cell clones; S.S. performed the generation and infection of human MDMs; C.S.-J. performed fluorescence-activated single cell sorting; U.M., K.R., and B. Fehse supported establishment of T-cell transduction with lentiviral LeGO vectors; B. Fleischer contributed to the concept and supervision; M.J. performed experiments, analyzed the data, supervised the study, and wrote the manuscript; and all authors read and approved all versions of the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Marc Jacobsen, Pediatric Infectious Diseases Group, Department of General Pediatrics, Neonatology, and Pediatric Cardiology, University Children's Hospital, Moorenstrasse 5, 40225 Duesseldorf, Germany; e-mail: marc.jacobsen@med.uni-duesseldorf.de.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal