Abstract
Natural killer (NK) cells elicit cytotoxicity against multiple myeloma (MM); however, MM cells express HLA class I molecules as ligands to NK cell inhibitory killer immunoglobulin-like receptors (KIRs) as a means of immunoevasion. KIR-ligand mismatch may improve outcomes in allogeneic transplantation for MM. Extrapolating on this concept, we conducted a phase 1 trial of IPH2101, an anti-KIR antibody, in patients with relapsed/refractory MM. IPH2101 was administered intravenously every 28 days in 7 dose–escalated cohorts (0.0003-3 mg/kg) for up to 4 cycles. Pharmacokinetic, pharmacodynamic, and correlative immunologic studies were completed. A total of 32 patients were enrolled. The biologic endpoint of full KIR2D occupancy across the dosing cycle was achieved without dose-limiting toxicity or maximally tolerated dose. One severe adverse event was noted. Pharmacokinetic and pharmacodynamic findings approximated preclinical predictions, and IPH2101 enhanced ex vivo patient–derived NK cell cytotoxicity against MM. No objective responses were seen. No evidence of autoimmunity was observed. These findings suggest that IPH2101 is safe and tolerable at doses that achieve full inhibitory KIR saturation, and this approach warrants further development in MM. This trial was registered at www.clinicaltrials.gov as #NCT00552396.
Introduction
Treatment options for multiple myeloma (MM), such as cytotoxic chemotherapy, radiation, and high-dose corticosteroids, have provided only modest benefit. The advent of novel drugs, however, such as the potent immune modulators thalidomide and lenalidomide, has revolutionized therapy and improved survival.1,2 Of note, immune modulators may exert anti-MM efficacy, in part, through favorable modulation of natural killer (NK) cell function against MM.3-5 NK cells have been shown to play an important role in the immune response to MM6-9 ; however, MM exhibits specific immunoevasive strategies to circumvent and attenuate NK-cell function.10-15
Unlike B and T cells, NK cells do not require costimulatory signals or gene rearrangement events to induce an immune response.16 Rather, NK cells initiate cytotoxicity via signaling through expression of activating and inhibitory surface receptors.17 HLA class I molecules on candidate target cells (particularly HLA-C) serve as ligands to killer immunoglobulin-like receptors (KIRs), an important class of inhibitory receptors on NK cells. Every NK cell capable of cytotoxicity must express at least 1 inhibitory KIR, and KIR-ligand–induced inhibitory signaling may prevent an immune response, even in the presence of an activating receptor-ligand interaction.16-18 This is especially relevant in MM as the disease expresses HLA class I molecules (and may up-regulate this expression) as an NK cell immunoevasive strategy.15
KIR-ligand mismatch in the donor → recipient direction in T-cell depleted, haploidentical stem cell transplantation can facilitate long-term remission in acute myeloid leukemia, and this may also occur in MM.19,20 Extrapolating on this concept, IPH2101 (formerly 1-7F9) is a human, IgG4 monoclonal antibody (mAb) against common inhibitory KIRs (KIR2DL-1, -2, and -3) which blocks KIR-ligand interaction and augments NK cell killing of autologous tumor cells.21 Herein, we report results of a phase 1, single-agent, dose-escalation trial of IPH2101 in relapsed/refractory MM with the primary objective of assessing the dose-limiting toxicity (DLT) and maximum tolerated dose for subsequent studies. IPH2101 was found to be safe and tolerable with achievement of the biologic endpoint of full KIR2D blockade over the dosing interval without DLT or identification of maximally tolerated dose. Correlative studies suggest that IPH2101 enhanced NK cell cytotoxicity against MM with no evidence of autoimmunity. Although no objective responses were documented by International Myeloma Working Group (IMWG) criteria,22 11 patients (34%) achieved stable disease on trial. These results support further development of IPH2101 as a novel therapy for MM. This trial was registered at www.clinicaltrials.gov as #NCT00552396.
Methods
Study objectives
The primary objective of the study was to determine the safety and tolerability of IPH2101 by NCI CTC Version 3.0 with particular attention to any evidence of autoimmunity. The secondary objectives were to assess the pharmacokinetic (PK) and pharmacodynamic (PD) parameters of IPH2101 and to determine any early signs of clinical efficacy. PD parameters included KIR occupancy on patient NK cells (an ex vivo assessment of the fraction of cell surface KIR occupied by IPH2101), immune cell regulation markers, immunophenotyping of NK- and T-cell subsets, analysis of cytokine profiles, functional assessment of ex vivo NK cell cytotoxicity against MM, and potential development of human antihuman antibodies against IPH2101.
Study population
Adult patients with relapsed/refractory MM who had received at least 1 prior line of therapy were eligible for inclusion with: measurable monoclonal protein, Eastern Cooperative Oncology Group performance of 0-2, adequate renal (serum creatinine < 1.5 × institutional upper limit of normal range) and hepatic function (total bilirubin < 1.5 × and AST < 3 × upper limit of normal range) and bone marrow reserve (absolute neutrophil count > 1.2 × 109/L and platelets > 70 × 109/L). Eligibility was also based on peripheral blood NK cell count initially at > 100 cells/mm3 and then > 50/mm3 and in vitro ability of IPH2101 to bind patient NK cells. Patients were excluded with a history of autoimmune disease, cytotoxic chemotherapy or radiotherapy within 28 days of screening, thalidomide or bortezomib within 14 days of screening, HIV, chronic hepatitis, or history of allogeneic transplantation. The protocol was amended to include a final extension cohort at the highest dose level (3 mg/kg) of patients limited to 1 prior line of therapy. All research reported in the manuscript was approved by Institutional Review Boards before initiation of work, and the study was conducted in accordance with the Declaration of Helsinki.
Study design
IPH2101 was administered intravenously once every 28 days over 7 cohorts at escalating doses: 0.0003, 0.003, 0.15, 0.075, 0.3, 1, and 3 mg/kg. The very low starting dose was predicted and extrapolated from a transgenic mice model21 to lead to detectable KIR occupancy without full saturation of KIR on patient NK cells. A standard 3 + 3 trial design was used with dosing cohorts added sequentially 1 month after completion of the previous cohort, following interim review of safety and tolerability data. Evidence of any DLT was assessed in an ongoing manner throughout the trial, and DLT was defined as any grade 3 event.
Response criteria
Responses were assessed by IMWG uniform criteria.22 Patients who received at least 1 dose of IPH2101 and had 2 subsequent evaluations of disease status were considered for response evaluation.
Correlative studies
PK plasma concentration-time data were analyzed by noncompartmental analysis with WinNonlin Enterprise Version 5.2 model 201 (bolus intravenous administration) and model 202 (constant rate intravenous infusion), including concentration at end of infusion, terminal half life (t1/2) clearance, apparent volume of distribution during the terminal phase, area under the curve from time 0 to infinity, and mean residence time. KIR occupancy was assessed by determining the amount of free (unbound) KIR on patient NK cells predosing and postdosing. The predose amount of free KIR (100% free KIR) was set to 0% occupancy. The KIR occupancy was also determined by a direct assessment of bound IPH2101 to the patient NK cells by the use of a labeled mouse anti-IgG4 mAb. An in vivo assessment of the fraction of cell surface KIR that was occupied by IPH2101 was calculated. The KIR occupancy (%) was calculated as: % free KIR2D = (100% × molecules of equivalent soluble fluorochrome (IPH2101-PE/CD56+/CD3− actual at time t)/molecules of equivalent soluble fluorochrome (IPH2101-PE/ CD56+/CD3− at predose) and % occupancy = 100% − % free KIR2D. KIR occupancy across dosing intervals was assessed as the biologic endpoint of the study with full KIR2D saturation defined as > 90%. Immune cell regulation markers, cytokine profiles (IFN-γ, IL-1, IL-2, IL-6, soluble IL-2 receptor, TNF-α, and MIP1β) were measured. For all patients, immunophenotyping of NK- and T-cell receptor expression included: CD25, CD69, CD107a, CD336, and perforin analyzed over serial time points. For patients in the extension cohort, immunophenotyping also included CD16, CD85, CD94, CD158a, CD158b, CD158i, CD159a, CD159c, CD161, CD224, CD226, CD314, CD335, CD337, and NKp80. In addition to the noncompartmental PK analysis, a population PK/PD model was developed to study the relationship between PK/PD, including the following variables: plasma concentration of IPH2101 at all time points, mean fluorescence intensity and molecules of equivalent soluble fluorochrome raw data from KIR binding assays, calculated % bound IPH2101, calculated % free KIR receptors, expression level of KIR-receptor subtypes at inclusion, number of NK cells and total lymphocytes, patient weight/BMI and sex, NK- and T-cell activation markers, markers for MM disease status, and results of NK cell ex vivo functional assay. The presence of NK cell precursors in peripheral blood was studied by methods previously described.23 To test NK-cell function, PBMCs were collected from patients before first dose and after each dose of IPH2101. NK cells in PBMCs were plated as effector cells in an ELISPOT cytotoxicity assay measuring granzyme B (GrB) production against the MHC class I expressing MM cell line RPMI8226 as targets (20:1 effector/target ratio) on MultiScreen 96-well plates (Millipore) per methods described previously.21,24 Effectors or targets alone served as controls. After 4-hour coculture, GrB spots were visualized and counted with an Immunospot Imaging Analyzer (Cellular Technology Ltd). Samples were run in triplicate, and intrapatient comparisons were made between baseline, pretreatment cytotoxicity, and NK cell cytotoxicity after IPH2101 infusion.
Statistical considerations
Continuous data were summarized using descriptive statistics; categorical data were summarized by number and percentage of events. Progression-free survival was calculated by the method of Kaplan-Meier. Student t test or ANOVA with repeated measures was used to study differences in GrB production in the functional assays as well as assessment of activation marker expression on effector cells before and after IPH2101 administration with P < .05 considered statistically significant. Correlations between IPH2101 binding and expression of NK-cell activation markers were analyzed by Pearson method with 2-tailed t test (P < .05 considered statistically significant).
Results
Patient characteristics
In total, 32 patients (19 male, 13 female; median age, 61 years; range, 35-83 years) were enrolled and received at least 1 dose of IPH2101. A total of 25 patients participated in the dose escalation, and 7 patients participated in the extension cohort at the end of the study. One patient in cohort 1 was replaced before dosing. Cohort 4 included n = 6 patients resulting from a serious adverse event (SAE) described in “Treatment, safety, and toxicity.” The median number of prior lines of therapy was 2 (range, 1-7), and average time from last treatment was 6.5 months (range, 1.2-32.9 months). All patients had received prior corticosteroid therapy and 81% had received prior alkylator–based therapy. Ninety-four percent of patients had also received prior lenalidomide, bortezomib, and/or thalidomide. Patients had an average of 2.2 years (range, 0.7-11 years) from diagnosis of MM to enrollment on trial. Cytogenetic information was available on n = 29 patients; 72% had “standard risk” cytogenetics.25 All patients, except 1 in the cohort 7 extension group, had actively progressing disease at time of enrollment. Patient demographics, disease, and prior treatment characteristics are summarized in Table 1.
Patient (n = 32) demographics, disease, and prior treatment characteristics
| Demographics . | Value . |
|---|---|
| Sex, female, no. (%) | 13 (41) |
| Median age, y (range) | 61 (35-83) |
| Prior lines of therapy, median (range) | 2 (1-7) |
| Corticosteroids | 100 (%) |
| Alkylating agent | 81 |
| Lenalidomide | 63 |
| Bortezomib | 59 |
| Autologous stem cell transplant | 59 |
| Thalidomide | 44 |
| Anthracycline | 38 |
| Other | 9 |
| Median time, diagnosis to enrollment, y, (range) | 2.2 (0.7-11) |
| Median time, last therapy to enrollment, mo, (range) | 6.5 (1.2-32.9) |
| International Staging System stage, % | |
| I | 81 |
| II | 6 |
| III | 13 |
| M-protein, % | |
| IgG | 59 |
| IgA | 28 |
| Light chain | 13 |
| Cytogenetics, % | |
| Standard risk | 72 |
| High risk | 28 |
| Demographics . | Value . |
|---|---|
| Sex, female, no. (%) | 13 (41) |
| Median age, y (range) | 61 (35-83) |
| Prior lines of therapy, median (range) | 2 (1-7) |
| Corticosteroids | 100 (%) |
| Alkylating agent | 81 |
| Lenalidomide | 63 |
| Bortezomib | 59 |
| Autologous stem cell transplant | 59 |
| Thalidomide | 44 |
| Anthracycline | 38 |
| Other | 9 |
| Median time, diagnosis to enrollment, y, (range) | 2.2 (0.7-11) |
| Median time, last therapy to enrollment, mo, (range) | 6.5 (1.2-32.9) |
| International Staging System stage, % | |
| I | 81 |
| II | 6 |
| III | 13 |
| M-protein, % | |
| IgG | 59 |
| IgA | 28 |
| Light chain | 13 |
| Cytogenetics, % | |
| Standard risk | 72 |
| High risk | 28 |
Treatment, safety, and toxicity
A total of 13 patients received 1 dose, 8 patients received 2 doses, 1 patient received 3 doses, and 10 patients completed all 4 planned doses. In total, 183 treatment-emergent adverse events (AEs) were reported, of which, 23 AEs were judged as “possibly,” “probably,” or “definitely” related to IPH2101. Most of these AEs were grade 1, self-limited, and related to infusion of IPH2101. A patient on cohort 4 with a 10-year history of MM and 7 prior lines of therapy experienced an SAE after the first administration of IPH2101. A potential DLT of acute renal failure requiring hemodialysis was judged as related to IPH2101 with a possible alternative explanation of disease progression. The cohort was expanded, however, and no further SAEs were observed. The most commonly reported treatment-emergent AE was fatigue (n = 10 patients), then chills, noncardiac chest pain, and pyrexia (n = 5 each). There were no deaths during the study, and no relationship between SAE occurrence or significant AEs and IPH2101 dose level was observed. Table 2 summarizes AEs possibly or probably related to IPH2101.
Adverse events “possibly” or “probably” related to IPH2101 cohort
| Event . | Grade . | 0.075 mg/kg . | 1 mg/kg . | 3 mg/kg . | Total . |
|---|---|---|---|---|---|
| Constitutional | |||||
| Pyrexia | 1 | 0 | 0 | 3 | 3 |
| Fatigue | 1 | 0 | 0 | 2 | 2 |
| Chills | 1 | 0 | 1 | 2 | 3 |
| Flushing | 1 | 0 | 0 | 1 | 1 |
| Headache | 1 | 0 | 1 | 1 | 2 |
| Cardiac | |||||
| Tachycardia | 2 | 1 | 0 | 0 | 1 |
| Gastrointestinal | |||||
| Abdominal pain | 1 | 1 | 0 | 0 | 1 |
| Nausea | 1 | 0 | 1 | 0 | 1 |
| Diarrhea | 1,2 | 0 | 0 | 2 | 2 |
| Laboratory values | |||||
| Neutrophil decrease | 3 | 0 | 0 | 1 | 1 |
| Creatinine increase | 4* | 1* | 0 | 0 | 1* |
| Renal/metabolism | |||||
| Acute renal failure | 3* | 1* | 0 | 0 | 1* |
| Hyperuricemia | 4* | 1* | 0 | 0 | 1* |
| Hyperkalemia | 4* | 1* | 0 | 0 | 1* |
| Event . | Grade . | 0.075 mg/kg . | 1 mg/kg . | 3 mg/kg . | Total . |
|---|---|---|---|---|---|
| Constitutional | |||||
| Pyrexia | 1 | 0 | 0 | 3 | 3 |
| Fatigue | 1 | 0 | 0 | 2 | 2 |
| Chills | 1 | 0 | 1 | 2 | 3 |
| Flushing | 1 | 0 | 0 | 1 | 1 |
| Headache | 1 | 0 | 1 | 1 | 2 |
| Cardiac | |||||
| Tachycardia | 2 | 1 | 0 | 0 | 1 |
| Gastrointestinal | |||||
| Abdominal pain | 1 | 1 | 0 | 0 | 1 |
| Nausea | 1 | 0 | 1 | 0 | 1 |
| Diarrhea | 1,2 | 0 | 0 | 2 | 2 |
| Laboratory values | |||||
| Neutrophil decrease | 3 | 0 | 0 | 1 | 1 |
| Creatinine increase | 4* | 1* | 0 | 0 | 1* |
| Renal/metabolism | |||||
| Acute renal failure | 3* | 1* | 0 | 0 | 1* |
| Hyperuricemia | 4* | 1* | 0 | 0 | 1* |
| Hyperkalemia | 4* | 1* | 0 | 0 | 1* |
Adverse event occurring in the same patient on cohort 4.
Binding of IPH2101 to effector cells
Binding of IPH2101 to NK and T cells was studied at baseline by flow cytometry. These results are summarized in Table 3 with HLA-C typing for each patient on study. The average peripheral NK cell count was 209/μL (± 130 SD), composing 14.7% (± 8%) of all lymphocytes. Of these, 44.6% (± 13.9%) of NK cells were IPH2101(+). IPH2101 binding was studied on patient NK cells during the trial as well. The percentage of IPH2101(+) NK cells did not appear to change over time (representative data from 3-mg/kg dose [cycle 1] cohort, Figure 1A). In most (n = 27) patients, the number of peripheral NK cells targeted by IPH2101 was higher than the number of T cells targeted.
NK- and T-cell populations and HLA-C typing for n = 32 patients in the study
| Dose . | Patient no. . | Peripheral T lymphocytes . | Peripheral NK lymphocytes . | NK/T . | HLA-C KIR ligand . | HLA-C KIR ligand . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All . | IPH2101(+) . | All . | IPH2101(+) . | |||||||||
| % . | abs/μL . | % . | abs/μL . | % . | abs/μL . | % . | abs/μL . | |||||
| 0.0003 | 1 | 58.2 | 606 | 20.9 | 127 | 28.6 | 295 | 58.7 | 173 | 1.4 | 05xx C2 | 07xx C1 |
| 0.003 | 2 | 80.1 | 1529 | 6.2 | 95 | 6.2 | 121 | 61.6 | 75 | 0.8 | 04xx C2 | 15xx C2 |
| 0.003 | 3 | 87.3 | 1955 | 0.8 | 16 | 7.9 | 185 | 23.5 | 43 | 2.8 | 01xx C1 | 07xx C1 |
| 0.015 | 4 | 77 | 907 | 11.7 | 106 | 23.9 | 275 | 68.6 | 189 | 1.8 | 04xx C2 | 07xx C1 |
| 0.015 | 5 | 59.5 | 970 | 6.9 | 67 | 25.7 | 380 | 46 | 175 | 2.6 | 07xx C1 | |
| 0.015 | 6 | 63.6 | 1732 | 2.2 | 38 | 11.9 | 324 | 41.8 | 135 | 3.6 | 04xx C2 | |
| 0.075 | 7 | 75.7 | 636 | 3.4 | 22 | 14.5 | 146 | 45 | 66 | 3.0 | 07xx C1 | 12xx C1 |
| 0.075 | 8 | 70.4 | 1072 | 0.4 | 4 | 12.1 | 182 | 55.4 | 101 | 23.5 | 04xx C2 | 15xx C2 |
| 0.075 | 9 | 57.2 | 927 | 1.9 | 18 | 5.1 | 109 | 33 | 36 | 2.0 | 03xx C1 | |
| 0.3 | 10 | 55.3 | 538 | 1.2 | 6 | 20.2 | 208 | 50 | 104 | 16.1 | 01xx C1 | 05xx C2 |
| 0.3 | 11 | 81.7 | 1222 | 3.2 | 39 | 6.9 | 117 | 18.8 | 22 | 0.6 | 01xx C1 | 07xx C1 |
| 1 | 12 | 76.8 | 1186 | 0.5 | 6 | 6.6 | 124 | 64.4 | 80 | 13.5 | 03xx C1 | 04xx C2 |
| 1 | 13 | 77.9 | 1033 | 12.5 | 129 | 14.8 | 199 | 51 | 101 | 0.8 | 07xx C1 | |
| 3 | 14 | 85.2 | 1046 | 3.1 | 32 | 5.7 | 71 | 35.5 | 25 | 0.8 | 03xx C1 | 07xx C1 |
| 3 | 15 | 51.1 | 631 | 1.9 | 12 | 21.6 | 295 | 45 | 133 | 11.1 | 04xx C2 | 12xx C1 |
| 3 | 16 | 55 | 911 | 0.6 | 5 | 17.9 | 300 | 43.7 | 131 | 24.0 | 03xx C1 | 04xx C2 |
| 3 | 17 | 49.7 | 653 | 1.8 | 12 | 21.4 | 287 | 28.8 | 83 | 7.0 | 05xx C2 | |
| 3 | 18 | 67.7 | 1147 | 0.6 | 7 | 17.5 | 338 | 41 | 139 | 20.1 | 07xx C1 | 15xx C2 |
| 3 | 19 | 72.5 | 1658 | 1.4 | 23 | 8.9 | 214 | 43.6 | 93 | 4.0 | 02xx C2 | 03xx C1 |
| 0.0003 | 20 | 72.3 | 664 | 2.3 | 15 | 11.6 | 108 | 22 | 24 | 1.6 | 03xx C1 | 06xx C1 |
| 0.0003 | 21 | 45.4 | 1137 | 3.5 | 40 | 15.5 | 368 | 78.7 | 290 | 7.3 | 03xx C1 | 04xx C2 |
| 0.0003 | 22 | 57.8 | 625 | 3.8 | 24 | 6.7 | 72 | 39 | 28 | 1.2 | 05xx C2 | 12xx C1 |
| 0.003 | 23 | 23.4 | 305 | 2.8 | 9 | 10.2 | 120 | 46.9 | 56 | 6.6 | 04xx C2 | 05xx C2 |
| 0.075 | 24 | 83.7 | 689 | 0.7 | 5 | 12.4 | 102 | 60.6 | 62 | 12.8 | 01xx C1 | |
| 0.075 | 25 | 75.5 | 435 | 18.9 | 82 | 17.6 | 95 | 38.7 | 37 | 0.4 | 04xx C2 | 06xx C1 |
| 0.075 | 26 | 47 | 580 | 5.3 | 31 | 12.4 | 139 | 39.7 | 55 | 1.8 | 01xx C1 | 07xx C1 |
| 0.3 | 27 | 56.1 | 1120 | 3.4 | 38 | 32.3 | 699 | 39.4 | 275 | 7.2 | 07xx C1 | 12xx C1 |
| 3 | 28 | 77.1 | 413 | 1.4 | 6 | 13.4 | 73 | 40.2 | 29 | 5.1 | 01xx C1 | 03xx C1 |
| 1 | 29 | 77.5 | 1652 | 1.6 | 26 | 6.8 | 163 | 48.6 | 79 | 3.0 | 01xx C1 | 16xx C1 |
| 3 | 30 | 86.3 | 1803 | 2.3 | 41 | 6.4 | 145 | 48.4 | 70 | 1.7 | 04xx C2 | 07xx C2 |
| 3 | 31 | 45.5 | 446 | 1.6 | 7 | 34.6 | 309 | 20.1 | 62 | 8.7 | 04xx C2 | |
| 3 | 32 | 60.6 | 546 | 1.1 | 6 | 12.8 | 116 | 48.6 | 56 | 9.4 | 12xx C1 | 16xx C1 |
| Mean | 65.9 | 962 | 4 | 34 | 14.7 | 209 | 44.6 | 95 | 6.4 | |||
| Median | 69.1 | 919 | 2.3 | 22 | 12.6 | 173 | 44.4 | 77 | 3.3 | |||
| SD | 15.1 | 451 | 5.1 | 36 | 8.0 | 130 | 13.9 | 67 | 6.7 | |||
| Dose . | Patient no. . | Peripheral T lymphocytes . | Peripheral NK lymphocytes . | NK/T . | HLA-C KIR ligand . | HLA-C KIR ligand . | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| All . | IPH2101(+) . | All . | IPH2101(+) . | |||||||||
| % . | abs/μL . | % . | abs/μL . | % . | abs/μL . | % . | abs/μL . | |||||
| 0.0003 | 1 | 58.2 | 606 | 20.9 | 127 | 28.6 | 295 | 58.7 | 173 | 1.4 | 05xx C2 | 07xx C1 |
| 0.003 | 2 | 80.1 | 1529 | 6.2 | 95 | 6.2 | 121 | 61.6 | 75 | 0.8 | 04xx C2 | 15xx C2 |
| 0.003 | 3 | 87.3 | 1955 | 0.8 | 16 | 7.9 | 185 | 23.5 | 43 | 2.8 | 01xx C1 | 07xx C1 |
| 0.015 | 4 | 77 | 907 | 11.7 | 106 | 23.9 | 275 | 68.6 | 189 | 1.8 | 04xx C2 | 07xx C1 |
| 0.015 | 5 | 59.5 | 970 | 6.9 | 67 | 25.7 | 380 | 46 | 175 | 2.6 | 07xx C1 | |
| 0.015 | 6 | 63.6 | 1732 | 2.2 | 38 | 11.9 | 324 | 41.8 | 135 | 3.6 | 04xx C2 | |
| 0.075 | 7 | 75.7 | 636 | 3.4 | 22 | 14.5 | 146 | 45 | 66 | 3.0 | 07xx C1 | 12xx C1 |
| 0.075 | 8 | 70.4 | 1072 | 0.4 | 4 | 12.1 | 182 | 55.4 | 101 | 23.5 | 04xx C2 | 15xx C2 |
| 0.075 | 9 | 57.2 | 927 | 1.9 | 18 | 5.1 | 109 | 33 | 36 | 2.0 | 03xx C1 | |
| 0.3 | 10 | 55.3 | 538 | 1.2 | 6 | 20.2 | 208 | 50 | 104 | 16.1 | 01xx C1 | 05xx C2 |
| 0.3 | 11 | 81.7 | 1222 | 3.2 | 39 | 6.9 | 117 | 18.8 | 22 | 0.6 | 01xx C1 | 07xx C1 |
| 1 | 12 | 76.8 | 1186 | 0.5 | 6 | 6.6 | 124 | 64.4 | 80 | 13.5 | 03xx C1 | 04xx C2 |
| 1 | 13 | 77.9 | 1033 | 12.5 | 129 | 14.8 | 199 | 51 | 101 | 0.8 | 07xx C1 | |
| 3 | 14 | 85.2 | 1046 | 3.1 | 32 | 5.7 | 71 | 35.5 | 25 | 0.8 | 03xx C1 | 07xx C1 |
| 3 | 15 | 51.1 | 631 | 1.9 | 12 | 21.6 | 295 | 45 | 133 | 11.1 | 04xx C2 | 12xx C1 |
| 3 | 16 | 55 | 911 | 0.6 | 5 | 17.9 | 300 | 43.7 | 131 | 24.0 | 03xx C1 | 04xx C2 |
| 3 | 17 | 49.7 | 653 | 1.8 | 12 | 21.4 | 287 | 28.8 | 83 | 7.0 | 05xx C2 | |
| 3 | 18 | 67.7 | 1147 | 0.6 | 7 | 17.5 | 338 | 41 | 139 | 20.1 | 07xx C1 | 15xx C2 |
| 3 | 19 | 72.5 | 1658 | 1.4 | 23 | 8.9 | 214 | 43.6 | 93 | 4.0 | 02xx C2 | 03xx C1 |
| 0.0003 | 20 | 72.3 | 664 | 2.3 | 15 | 11.6 | 108 | 22 | 24 | 1.6 | 03xx C1 | 06xx C1 |
| 0.0003 | 21 | 45.4 | 1137 | 3.5 | 40 | 15.5 | 368 | 78.7 | 290 | 7.3 | 03xx C1 | 04xx C2 |
| 0.0003 | 22 | 57.8 | 625 | 3.8 | 24 | 6.7 | 72 | 39 | 28 | 1.2 | 05xx C2 | 12xx C1 |
| 0.003 | 23 | 23.4 | 305 | 2.8 | 9 | 10.2 | 120 | 46.9 | 56 | 6.6 | 04xx C2 | 05xx C2 |
| 0.075 | 24 | 83.7 | 689 | 0.7 | 5 | 12.4 | 102 | 60.6 | 62 | 12.8 | 01xx C1 | |
| 0.075 | 25 | 75.5 | 435 | 18.9 | 82 | 17.6 | 95 | 38.7 | 37 | 0.4 | 04xx C2 | 06xx C1 |
| 0.075 | 26 | 47 | 580 | 5.3 | 31 | 12.4 | 139 | 39.7 | 55 | 1.8 | 01xx C1 | 07xx C1 |
| 0.3 | 27 | 56.1 | 1120 | 3.4 | 38 | 32.3 | 699 | 39.4 | 275 | 7.2 | 07xx C1 | 12xx C1 |
| 3 | 28 | 77.1 | 413 | 1.4 | 6 | 13.4 | 73 | 40.2 | 29 | 5.1 | 01xx C1 | 03xx C1 |
| 1 | 29 | 77.5 | 1652 | 1.6 | 26 | 6.8 | 163 | 48.6 | 79 | 3.0 | 01xx C1 | 16xx C1 |
| 3 | 30 | 86.3 | 1803 | 2.3 | 41 | 6.4 | 145 | 48.4 | 70 | 1.7 | 04xx C2 | 07xx C2 |
| 3 | 31 | 45.5 | 446 | 1.6 | 7 | 34.6 | 309 | 20.1 | 62 | 8.7 | 04xx C2 | |
| 3 | 32 | 60.6 | 546 | 1.1 | 6 | 12.8 | 116 | 48.6 | 56 | 9.4 | 12xx C1 | 16xx C1 |
| Mean | 65.9 | 962 | 4 | 34 | 14.7 | 209 | 44.6 | 95 | 6.4 | |||
| Median | 69.1 | 919 | 2.3 | 22 | 12.6 | 173 | 44.4 | 77 | 3.3 | |||
| SD | 15.1 | 451 | 5.1 | 36 | 8.0 | 130 | 13.9 | 67 | 6.7 | |||
Serial NK cell assessments in patients on study over time. (A) Normalized % IPH2101(+) NK cells (compared with predose) over time in cycle 1. This did not appear to change over time or be influenced by baseline peripheral NK cell count. (B) Representative finding of NK cell and T cell % KIR occupancy from a patient on cohort 4 (0.075 mg/kg). In most patients, the number of T cells recognized by IPH2101 was too low for evaluation; however, in n = 9 evaluable patients, % KIR occupancy between NK and T cells appeared closely correlated as shown. Peripheral absolute (C) and percent (D) NK cell counts over time (normalized to NK cell count obtained just before first administration of IPH2101). Neither absolute nor percent NK cell count appeared to change significantly from baseline over the duration of IPH2101 therapy. (Note patient n per time points shown varies as not all patients received all 4 cycles of planned therapy.)
Serial NK cell assessments in patients on study over time. (A) Normalized % IPH2101(+) NK cells (compared with predose) over time in cycle 1. This did not appear to change over time or be influenced by baseline peripheral NK cell count. (B) Representative finding of NK cell and T cell % KIR occupancy from a patient on cohort 4 (0.075 mg/kg). In most patients, the number of T cells recognized by IPH2101 was too low for evaluation; however, in n = 9 evaluable patients, % KIR occupancy between NK and T cells appeared closely correlated as shown. Peripheral absolute (C) and percent (D) NK cell counts over time (normalized to NK cell count obtained just before first administration of IPH2101). Neither absolute nor percent NK cell count appeared to change significantly from baseline over the duration of IPH2101 therapy. (Note patient n per time points shown varies as not all patients received all 4 cycles of planned therapy.)
The average peripheral T-cell count was 962/μL (± 451/μL), composing 65.9% (± 15.1%) of all lymphocytes. Of these, 4% (± 5.1%) of T cells were IPH2101(+), and for most patients, this percentage was too low to evaluate accurately over time. In n = 9 evaluable patients, the percent KIR occupancy on NK cells and T cells was generally closely correlated (representative finding, Figure 1B).
Circulating NK cell numbers (absolute and percent) did not appear to change over the course of the trial (Figure 1C-D). No changes in NK cell precursors were observed (data not shown).
PK and PD data
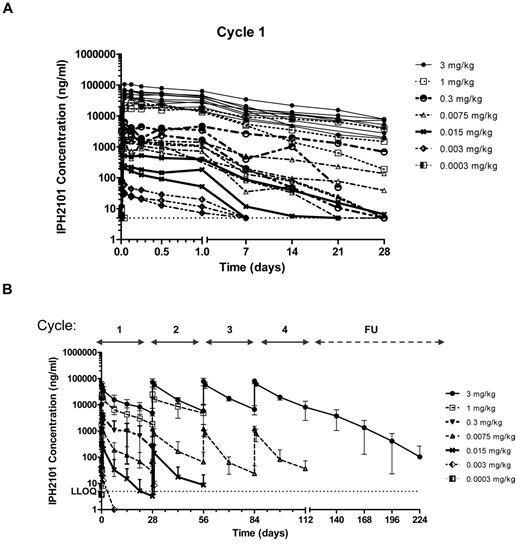
The population PK analysis included 375 observations (346 measurable) from n = 28 patients. IPH2101 concentrations were measured before infusion, and at 0.167, 1, 3, 6, 12, and 24 hours postdosing, then at days 7, 14, and 21 after the first dose administration. For subsequent cycles, samples were drawn before infusion, at the end of infusion, and at 24 hours and 14 days after infusion. Analysis of PK data demonstrated a clear relationship between dose and drug concentration. The increase in t1/2 was consistent with changes in the disposition from nonlinear driven to linear-driven clearance mechanisms. Mean elimination t1/2 at the higher dose levels (1 and 3 mg/kg) was ∼ 11 days, consistent with the t1/2 of immunoglobulin G (IgG) and other mAbs. A dose-proportional increase in Cmax was observed at cycles 2 and 3, and the terminal phase appeared consistent across dose levels suggesting limited distribution of IPH2101 outside the vascular space. A population PK analysis showed that a 2-compartment model with first-order elimination was found to describe data with dose–dependent clearance, such that clearance decreased with increasing doses. The terminal t1/2 at the highest dose was determined to be 18 days. Figure 2 shows IPH2101 concentration in cycle 1 by cohort (Figure 2A) and IPH2101 concentration across cycles by cohort (Figure 2B).
IPH2101 pharmacokinetics over time. Pharmacokinetic data are shown as IPH2101 concentration (ng/mL) over time for all treated subjects by dose cohorts in cycle 1 (A) and across all cycles (B). A clear relationship between dose and concentration over time was observed.
IPH2101 pharmacokinetics over time. Pharmacokinetic data are shown as IPH2101 concentration (ng/mL) over time for all treated subjects by dose cohorts in cycle 1 (A) and across all cycles (B). A clear relationship between dose and concentration over time was observed.
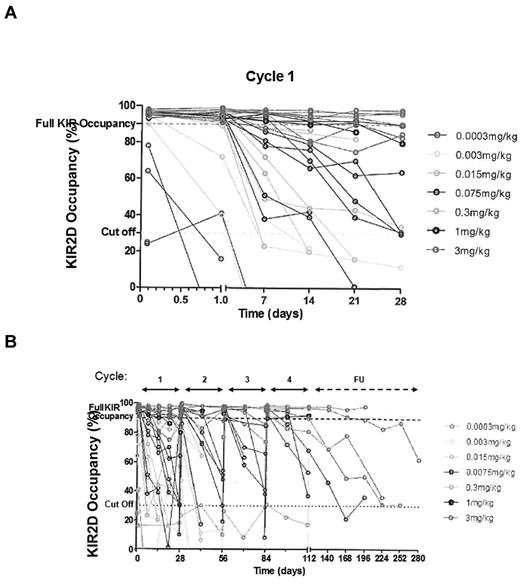
The PD end point of KIR occupancy was assessed before infusion and at 2 and 24 hours after the start of each cycle. In addition, samples were drawn at 7, 14, and 21 days after the first dose, at 14 days after subsequent doses, and monthly in follow-up from the fourth cycle. A population PD analysis showed an effect compartment Emax model using IPH2101 concentrations as predicted by the PK model adequately described the relationship. The EC50 defined as the serum drug concentration to reach 50% of Emax (50% of KIR occupancy) was estimated to be 34.2 ng/mL. Based on simulations using the final population PK/PD model, the highest dose level (3 mg/kg) achieved > 90% KIR occupancy for the full 4 cycle treatment duration whereas a 2-mg/kg dose would be predicted to achieve > 90% KIR occupancy over most of the dosing interval, as well. KIR occupancy data are shown in Figure 3A and B.
IPH2101 pharmacodynamics over time. Pharmacodynamic data are shown as KIR occupancy (%) over time for all treated subjects by dose cohorts in cycle 1 (A) and across all cycles (B). Full KIR2D occupancy was defined as > 90%, and the cut-off value was defined as 30%. A clear relationship between dose and KIR occupancy over time was observed.
IPH2101 pharmacodynamics over time. Pharmacodynamic data are shown as KIR occupancy (%) over time for all treated subjects by dose cohorts in cycle 1 (A) and across all cycles (B). Full KIR2D occupancy was defined as > 90%, and the cut-off value was defined as 30%. A clear relationship between dose and KIR occupancy over time was observed.
Immunomodulation by IPH2101
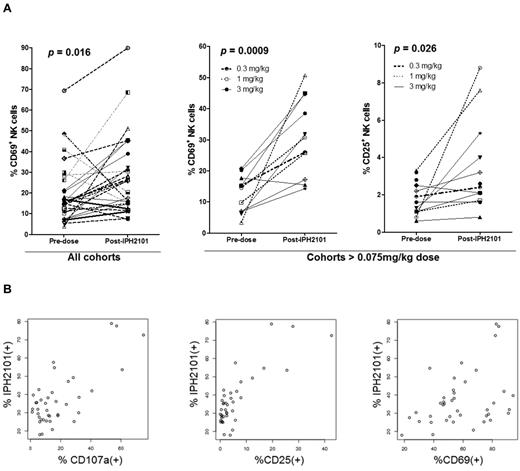
Serial assessments were performed to evaluate cytokine profiles and T- and NK-cell subsets in patients on trial. No significant changes were seen in IFN-γ, TNF-α, MIP-1β, IL-1β, IL-2, or IL-6 levels as no significant data over limit of detection were determined. NK cell expression of CD69, CD25, and CD107a was studied after first administration of IPH2101. As shown in Figure 4A assessing all patients treated, CD69 expressed increased after IPH2101 dosing (P = .016). With regard to the patients who received doses > 0.075 mg/kg, both CD69 (P = 0.0009) and CD25 (P = .026) expression were increased. Samples from n = 8 patients enrolled on the final 3-mg/kg extension cohort to examine any correlation between IPH2101 binding and NK-cell activation. In these analyses, the percentage of IPH2101(+) NK cells appeared to correlate with percentage of NK cells expressing CD107a (P = .000000093), CD25 (P = .00000000012), and CD67 (P = .01, Figure 4B). No statistically significant differences were observed in T-cell expression of activation markers or relationship to IPH2101 binding after IPH2101 administration (data not shown).
Evidence of NK cell activation by IPH2101. (A) Left panel: Increased expression of CD69 24 hours after first administration of IPH2101 compared with baseline (P = .016) for all patients. Center and right panels: Increased expression of CD69 (P = .0009) and CD25 (P = .026) in patients (n = 11) receiving IPH2101 doses > 0.075 mg/kg, suggesting a dose-response effect regarding NK-cell activation (as this was not observed in patients who received < 0.075 mg/kg). (B) IPH2101 binding appears to correlate with NK-cell activation. Data shown are from n = 8 evaluable subjects on the 3 mg/kg extension cohort where the percentage of IPH2101(+) NK cells appears to correlate with NK cell expression of CD107a (P = .000000093), CD25 (P = .00000000012), and CD69 (P = .01).
Evidence of NK cell activation by IPH2101. (A) Left panel: Increased expression of CD69 24 hours after first administration of IPH2101 compared with baseline (P = .016) for all patients. Center and right panels: Increased expression of CD69 (P = .0009) and CD25 (P = .026) in patients (n = 11) receiving IPH2101 doses > 0.075 mg/kg, suggesting a dose-response effect regarding NK-cell activation (as this was not observed in patients who received < 0.075 mg/kg). (B) IPH2101 binding appears to correlate with NK-cell activation. Data shown are from n = 8 evaluable subjects on the 3 mg/kg extension cohort where the percentage of IPH2101(+) NK cells appears to correlate with NK cell expression of CD107a (P = .000000093), CD25 (P = .00000000012), and CD69 (P = .01).
IPH2101 enhanced patient–derived NK cell cytotoxicity against MM
Patient–derived NK cell cytotoxicity was studied ex vivo against the MHC class I expressing MM cell line RPMI 8226. PBMCs were obtained from patients before first IPH2101 dose and at 24 hours after dose. In 6 of 8 patients studied, IPH2101 enhanced ex vivo NK cell cytotoxicity an average of 3.5-fold (SD ± 1.8-fold, P = .01) from baseline against MM tumor cell targets (Figure 5). Target cells and IPH2101-treated patient effector cells were cultured in independently as control conditions, as well. IPH2101-treated patient cells showed no increase in granzyme B production in the absence of targets (dark gray bars, Figure 5A), and target cells alone produced no granzyme B (data not shown). Subsequently, PBMCs were obtained from patients before each additional dose of IPH2101, and over the course of the extension trial, NK cell cytotoxicity was enhanced 2.34-fold (± 0.8-fold, P = .04) against MM targets over baseline (data from n = 5 available patient samples shown in Figure 5B).
Evidence of NK cell cytotoxicity by IPH2101. (A) In n = 6 of 8 subjects evaluated, IPH2101 appeared to increase NK cell cytotoxicity against MM. Shown are ex vivo NK cell cytotoxicity results measuring NK cell production of GrB by ELISPOT in coculture with RPMI 8226 MM cell line targets. The light gray condition shows GrB production against MM cell line targets before first dose of IPH2101. The black bar represents GrB production 24 hours after first dose of IPH2101. As a control for the possibility of spontaneous activation; and dark gray bar, effector cell GrB production after IPH2101 dosing in the absence of targets. All pair-wise comparisons between predose and postdose and effectors alone versus postdose are statistically significant: *P < .05. (B) NK cell cytotoxicity for n = 5 patients who received repeated doses of IPH2101 on the extension cohort. *GrB production significantly greater than baseline.
Evidence of NK cell cytotoxicity by IPH2101. (A) In n = 6 of 8 subjects evaluated, IPH2101 appeared to increase NK cell cytotoxicity against MM. Shown are ex vivo NK cell cytotoxicity results measuring NK cell production of GrB by ELISPOT in coculture with RPMI 8226 MM cell line targets. The light gray condition shows GrB production against MM cell line targets before first dose of IPH2101. The black bar represents GrB production 24 hours after first dose of IPH2101. As a control for the possibility of spontaneous activation; and dark gray bar, effector cell GrB production after IPH2101 dosing in the absence of targets. All pair-wise comparisons between predose and postdose and effectors alone versus postdose are statistically significant: *P < .05. (B) NK cell cytotoxicity for n = 5 patients who received repeated doses of IPH2101 on the extension cohort. *GrB production significantly greater than baseline.
Evaluation of clinical efficacy
Safety and tolerability were the primary end points of the trial. Patients were eligible for response evaluation by IMWG criteria, and no objective responses were observed.22 Eleven (34%) patients achieved a best response of stable disease.
Discussion
That NK cells play an important role in the immune response to MM was first reported > 25 years ago6 ; however, from 1971 until 2000, the overall survival of newly diagnosed patients with MM did not appreciably change.1 In the last decade, the advent of novel therapies (thalidomide, lenalidomide, and bortezomib) that may exert anti-MM efficacy, in part, through favorable modulation of the NK cell versus MM effect has been associated with a 50% improvement in overall survival.1
The expression of HLA class I molecules by MM tumor cells may represent a critical immunoevasive strategy.15 These antigens serve as ligands to inhibitory KIR displayed by NK cells, and disruption of the KIR/ligand relationship may be associated with improved outcomes in the T cell–depleted, allogeneic stem cell transplant setting for patients with MM.15,20
Extrapolating on this principle, IPH2101 is a human IgG4 mAb against inhibitory KIR and functions to block the inhibitory KIR-ligand relationship to recover or augment NK-cell function against tumor cells.21 IPH2101 is a nondepleting antibody and is not bound by Fc receptors. Preclinical data demonstrate that augmented NK-cell function is directed specifically against malignant cells and not normal cells,21,26 and no evidence of autoimmunity was observed in the present trial. This is possibly because of tumor cells expressing ligands for activating NK cell receptors that are not expressed on normal tissues.26 Thus, by blocking inhibitory signaling, IPH2101 tips the balance between activating and inhibitory signals toward favoring the induction of an NK cell immune response against tumor cell targets.
In the present work, we identified doses of IPH2101, which confer full KIR2D occupancy in vivo across the dosing interval with no concomitant DLT or identification of a maximally tolerated dose. The safety profile of IPH2101 is acceptable. With one exception, adverse events deemed related to study drug were mild and transient and, for the most part, associated with self-limited infusion reactions.
PK and PD data closely approximate preclinical models, and a strong relationship between Cmax and KIR occupancy was observed. In addition, IPH2101 appeared to enhance ex vivo patient–derived NK cell cytotoxicity against MM tumor cell targets. As a murine model of NK cell development has demonstrated the requirement of inhibitory receptor expression for functional competency,27 NK cell precursor subsets were analyzed in peripheral blood of patients on study, and no deleterious effect was observed on peripheral NK cell counts or NK cell development in response to IPH2101.
All patients on the present trial had received prior therapy with corticosteroids, which irreversibly blunt NK-cell function in patients with MM.28 Most patients had also received prior immune suppressive treatments, including alkylator agent–based therapy, and many had received prior anthracycline treatment as well. In addition, advanced MM, per se, confers progressive immune dysfunction, and patients had an average duration of disease of > 2 years on trial. Nonetheless, IPH2101 therapy appeared to be associated with NK-cell activation, and NK cell cytotoxicity against autologous MM target cells appeared to be enhanced by IPH2101.
Recent data suggest that releasing the immune system from inhibitory signaling may be an especially potent form of anticancer therapy. For instance, an mAb against cytotoxic T-lymphocyte antigen 4 was recently shown to improve survival in patients with previously treated metastatic melanoma.29 In addition, therapy designed to limit or ablate regulatory T-cell function has also demonstrated benefit.30 Finally, an mAb against Programmed-death receptor 1 also showed clinical benefit in 33% of patients with advanced hematologic malignancies in a phase 1 dose-escalation trial.31 In this context, and in light of prior, compelling results in the setting of haploidentical allogeneic stem cell transplantation, disruption of the KIR-ligand relationship holds promise as a novel strategy to release the immune system from inhibitory signaling and confer tumor-specific, NK cell-mediated immunity in the autologous setting, potentially achieving a graft versus MM effect in the absence of allogeneic transplantation.19-21
IPH2101 is a first-in-class, anti-KIR mAb capable of recovering and enhancing NK-cell function against autologous tumor cells.21 Herein we show that IPH2101 is safe and tolerable in patients with advanced MM with preliminary evidence of disease stabilization in some patients. These results lend support to further development of anti-KIR mABs as a novel anti-MM therapy.
Portions of this work were presented at the American Society of Clinical Oncology Annual Meeting (June 8, 2009; June 14, 2010), the American Society of Hematology Annual Meeting (December 9, 2009; December 4, 2010), and the International Myeloma Workshop (May 4, 2011).
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Stephane Blanchet for his assistance in executing the clinical trial.
This work was supported by Conquer Cancer Foundation of the American Society of Clinical Oncology (Career Development Award 60019229; D.M.B.), Pelotonia IDEA Award (D.M.B.), National Cancer Institute (P01CA095426; M.A.C. and D.M.B.), and Multiple Myeloma Opportunities for Research and Education (D.M.B., C.C.H., and Y.E.).
National Institutes of Health
Authorship
Contribution: D.M.B. designed and conducted research, served as national principal investigator, and wrote the manuscript; C.C.H., S.P., A.S., S.J., R.A., C.B., P.A., Y.E., and S.S.F. performed clinical and/or correlative research; J.T. and M.A.C. designed research; and all authors read and approved the final manuscript.
Conflict-of-interest disclosure: D.M.B. and S.S.F. have received research funding from Innate Pharma. P.A. and J.T. are employees of Innate Pharma. The remaining authors declare no competing financial interests.
Correspondence: Don M. Benson Jr, Division of Hematology, 898 Biomedical Research Tower, The Ohio State University Comprehensive Cancer Center, 460 W 12th Ave, Columbus, OH 43210-1240; e-mail: don.benson@osumc.edu.



This feature is available to Subscribers Only
Sign In or Create an Account Close Modal