In this issue of Blood, Bolaños-Meade et al report promising results using a pioneering HLA-haploidentical bone marrow transplantation protocol in adults with sickle cell disease (SCD), including nonmyeloablative conditioning and post-transplantation high-dose cyclophosphamide.1
Despite considerable progress made in the management of SCD such as the prevention of pneumococcal infections, the introduction of hydroxyurea therapy, and the early detection of cerebral vasculopathy with transcranial Doppler (TCD), SCD remains a disease with high risk of morbidity and early death. Hematopoietic stem cell transplantation (HSCT) is currently the only curative treatment for SCD. Nevertheless, its use has been limited by the lack of available donors, and also by the risks of transplant-related mortality (TRM), graft-versus-host disease (GVHD), infertility, and its cost for a disease that principally affects patients living in countries with low gross domestic product.
For several years Luznik et al have been developing the interesting concept of tolerance induction by using early post-transplantation high doses of cyclophosphamide, which kill proliferative alloreactive T cells while preserving resting nonalloreactive T cells.2 Bolaños-Meade et al report here their experience with 14 SCD patients who received a transplant using a haplo-identical donor. Their results will allow the expansion of the donor pool for most SCD patients in the future as almost every patient has a haploidentical-matched parent or sibling.
In the US and European countries, myeloablative HLA geno-identical transplantation is now a well-established treatment option in symptomatic children.3,4 Published results of approximately 200 patients reported an 85% chance of cure with 7% TRM and 8% rejection risk.2-4 However, as demonstrated in a French study,4 the addition of rabbit antithymocyte globulin (ATG) allows for a large decrease in the risk of rejection, resulting in significant improvement in results over time with a 95% chance of cure. These results have now been confirmed by our experience with 120 more young patients who have received a transplant since 2000.5
To extend the possibility of cure to SCD adults with organ dysfunction, nonmyeloablative conditioning regimens were proposed, but initial trials failed to induce stable donor engraftment6 despite the HLA-identical context of the transplants. This underlines the singularity of transplantation for SCD where the recipient is fully immunocompetent and has a proliferative bone marrow, and GVHD has no utility. More recently a nonmyeloablative conditioning regimen based on alemtuzumab (Campath), low-dose total body irradiation (TBI), and transplantation with peripheral blood stem cells (PBSCs) from an HLA-identical donor followed by sirolimus was successfully applied to 10 adults.7 Remarkably, no TRM or GVHD occurred, and donor engraftment was observed in 9 of 10 recipients. Sirolimus (rapamycin), unlike cyclosporine, does not block T-cell activation and promotes T-cell tolerance.7 However, donor/recipient pairs with ABO incompatibility were excluded from this trial. Of note, despite frequent stable mixed chimerism in these studies,4,5,7 erythropoiesis was of donor origin, resulting in operational cure of the patient.
In countries with high standards of care the major obstacle to wide application of transplantation in SCD remains the paucity of HLA-identical related donors because less than 25% of children have a healthy familial-identical donor. However, as HSCT is not an urgent indication in SCD as is the case in malignant diseases, the chances of having an identical donor will increase during the infancy of the sick SCD child if the providers discuss HSCT early, and propose prenatal or preimplantatory diagnosis and sibling cord blood cryopreservation when parents are expecting another child. Nevertheless, HLA-identical unrelated donors from registries remain very difficult to find for minorities,8 and unrelated cord blood transplants (CBTs) gave disappointing results in SCD with high risk of rejection and GVHD, resulting in premature closing of such trials.9
In contrast, nearly all patients have a related HLA-haploidentical donor. This is why the pioneering work by Bolaños-Meade and colleagues described here brings new hope of long-term cure for a large number of patients suffering from severe forms of SCD. Most importantly, the authors show that by using high doses of cyclophosphamide after transplantation, none of the patients suffered severe GVHD or complications of severe immunodeficiency, which represent the traditional obstacles to mismatch transplantation. The huge rejection rate remains a major problem: 43% or 50%, depending on the classification of 1 patient with very low chimerism; however, all patients survived the procedure, and those who rejected their graft experienced autologous reconstitution and the return of their disease. Even though one cannot disregard that patients received ATG with Fludarabin, low-dose TBI, high-dose cyclophosphamide, and immunosuppressive drugs and experienced neutropenia for a median of 24 days, a 50% rejection rate can be considered acceptable when it relates to patients with life-threatening SCD complications, a new paradigm in nonmyeloablative BMT for nonmalignant diseases.
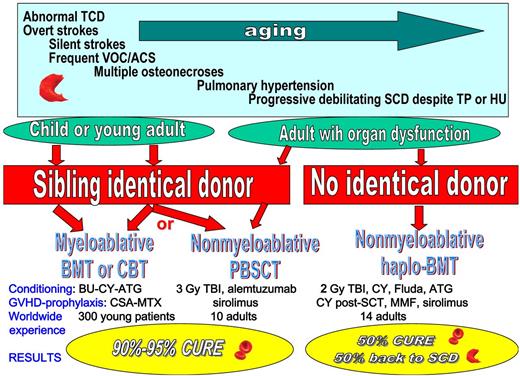
Despite remaining an unpredictable disease, several severity criteria have been identified during the past years that justify the research of available donors, including severe anemia (< 7 g/dL), abnormal TCD, overt and silent strokes, frequent vaso-occlusive crises and acute chest syndromes, multiple osteonecroses, and pulmonary hypertension occurring with time. In particular, by age 14, 50% of SCD patients are at risk of cerebral vasculopathy,10 which is responsible for declining cognitive performance. Myeloablative BMT or CBT should be considered as standard care for children who require intensive therapies (transfusion program or hydroxyurea) and have an available geno-identical sibling donor. Nonmyeloablative identical HSCT using PBSCs as a cell source (National Institutes of Health trial7 ) should be preferred for adult patients with organ dysfunction, and haploidentical BMT could be proposed to patients with no identical donor and progressively debilitating SCD despite transfusion program or hydroxyurea therapy, provided that the interesting results obtained by Bolaños-Meade et al are confirmed by other studies (see figure). Haploidentical BMT could be proposed to a larger number of adult patients as most will have a donor, as long as they are fully informed on the risks of return of the disease.
The choice of HSCT protocol will depend on the availability of an identical donor, the patient's age, the severity of SCD, and its consequences on the degree of organ dysfunction. TCD indicates transcranial Doppler; VOC, vaso-occlusive crises; ACS, acute chest syndrome; TP, transfusion program; HU, hydroxyurea; BMT, bone marrow transplantation; CBT, cord blood transplantation; PBSCT, peripheral blood stem cell transplantation; TBI, total body irradiation; Fluda, Fludarabin; MMF, mycophenolate mofetil; BU, busulfan; CY, cyclophosphamide; MTX, methotrexate; and ATG, antithymoglobulin.
The choice of HSCT protocol will depend on the availability of an identical donor, the patient's age, the severity of SCD, and its consequences on the degree of organ dysfunction. TCD indicates transcranial Doppler; VOC, vaso-occlusive crises; ACS, acute chest syndrome; TP, transfusion program; HU, hydroxyurea; BMT, bone marrow transplantation; CBT, cord blood transplantation; PBSCT, peripheral blood stem cell transplantation; TBI, total body irradiation; Fluda, Fludarabin; MMF, mycophenolate mofetil; BU, busulfan; CY, cyclophosphamide; MTX, methotrexate; and ATG, antithymoglobulin.
While HSCT brings considerable hope to SCD patients, we have to keep in mind that such procedures are not accessible to the majority of patients living in African countries; thus, the development of new drugs should still be encouraged. However, even if efficient new drugs become available, it will be necessary to develop prospective trials comparing their long-term efficacy with that of HSCT.
Conflict-of-interest disclosure: The authors declare no competing financial interests. ■