In this issue of Blood, Greve et al report that ovaries from leukemia patients in complete remission do not appear to contain viable malignant cells, in contrast to ovarian tissue retrieved before chemotherapy.1
This paper raises a number of questions because 3 different teams (Meirow et al,2 Rosendahl et al,3 and Dolmans et al4 ) have so far demonstrated by PCR that ovarian tissue from leukemia patients is positive for malignant cells in more than 50% of cases.
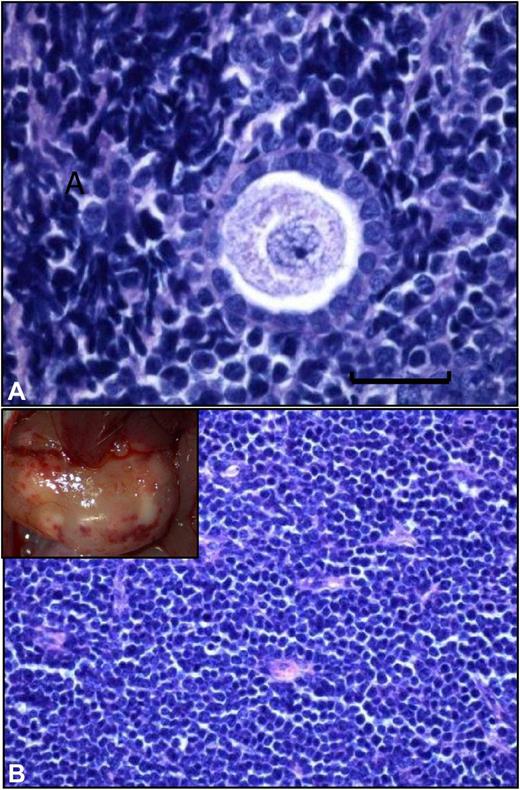
We have previously reported that xenografting of cryopreserved ovarian tissue from leukemia patients (chronic and acute leukemia) resulted in development of leukemic tumors (see figure).4 Differences between the results of these 2 studies may be explained, at least partially, by the fact that of the 25 women Greve et al studied, 17 had received chemotherapy and were in remission, 4 had received initial treatment but had not achieved remission, 3 had chronic-phase chronic myeloid leukemia, and 1 had an unknown remission status at the time of ovarian biopsy and cryopreservation.1,4
Grafted frozen-thawed ovarian tissue from a leukemia patient. (A) Histologic analysis of a frozen-thawed ovarian fragment from a leukemia patient recovered from a mouse after 6 months' xenografting. Normal ovarian stroma is no longer present, and a human follicle encircled by a large number of proliferating malignant lymphocytes can be seen (scale bar = 20 μm). (B) Large, white, burgeoning mass that corresponded to massive peritoneal invasion by leukemic cells.
Grafted frozen-thawed ovarian tissue from a leukemia patient. (A) Histologic analysis of a frozen-thawed ovarian fragment from a leukemia patient recovered from a mouse after 6 months' xenografting. Normal ovarian stroma is no longer present, and a human follicle encircled by a large number of proliferating malignant lymphocytes can be seen (scale bar = 20 μm). (B) Large, white, burgeoning mass that corresponded to massive peritoneal invasion by leukemic cells.
In the study by Greve et al, of the 7 patients with a known marker and who had already received chemotherapy at the time of ovarian cryopreservation, 4 showed PCR-positive ovarian tissue (2 with chronic leukemia and 2 in remission with acute leukemia). Even if xenografts from these patients failed to induce tumoral development, it cannot be excluded that cancer cells may be present in other tissue pieces.
Nine of the initial 45 acute or chronic leukemia patients identified with cryopreserved ovaries had already died before initiation of this study. Unfortunately, the study of the preserved ovarian tissue from the dead women was not authorized by the local ethics committee.1 It would have been extremely valuable to confirm the presence or absence of malignant cells in the ovarian tissue of these patients, as some were likely in complete remission at the time of the cryopreservation procedure. These patients probably presented with a more aggressive form of the disease and were thus most at risk of carrying malignant cells in their ovarian tissue.
Even if some of the results from Greve et al are reassuring and demonstrate that ovarian tissue taken from leukemia patients in complete remission after chemotherapy does not induce tumors in a xenograft model, the presence of positive PCR should lead us to interpret these results with caution.
It is too early to be sure that frozen-thawed ovarian tissue from leukemia patients in complete remission contains nonviable malignant cells or too few cells to reintroduce cancer. From a hematologic point of view, blasts may be more fragile than ovarian tissue and not able to withstand the cryopreservation procedure. Moreover, if a few viable malignant cells remain in the tissue, time may be required before they become detectable. Late relapse can sometimes occur more than 2 years after remission is induced.
In conclusion, like Greve and colleagues, our team believes that the risk of cryopreserved ovarian tissue from leukemia patients in complete remission containing malignant cells is low. However, it cannot be completely excluded. This study should be confirmed by other teams before ovarian transplantation is considered risk-free in these patients. We agree with Greve et al that alternative methods like in vitro maturation or isolated follicle transplantation should be evaluated for fertility preservation in leukemia patients.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal