Abstract
Abstract  905
905
Though dose-intensive strategies using chemoimmunotherapy have significantly improved mantle cell lymphoma (MCL) outcomes with prolonged progression-free survival (PFS), most patients still relapse over time. In the relapsed setting, MCL patients often develop chemoresistance and have a poor overall prognosis. The immunomodulatory agent lenalidomide has demonstrated tumoricidal and antiproliferative effects in MCL and clinical activity and safety in multiple phase II studies in aggressive non-Hodgkin's lymphoma. The objective of the MCL-001 “EMERGE” study was to examine the safety and efficacy of single-agent lenalidomide in subjects with MCL who relapsed or were refractory to bortezomib.
This phase II, multicenter, single-arm, open-label study examined single-agent lenalidomide administered at 25 mg/d PO on days 1–21 of a 28-day cycle until disease progression, unacceptable toxicity, or voluntary withdrawal. The subjects were required to have had prior treatment with rituximab, cyclophosphamide and anthracycline (or mitoxantrone), and to have relapsed or progressed (<12 months) after or were refractory to bortezomib. The primary endpoints were overall response rate (ORR) and duration of response (DOR); secondary endpoints included complete response (CR), PFS, time to progression (TTP), overall survival (OS) and safety. Efficacy data were measured by investigators and an independent central review committee according to modified International Working Group criteria and analyzed by SAS.
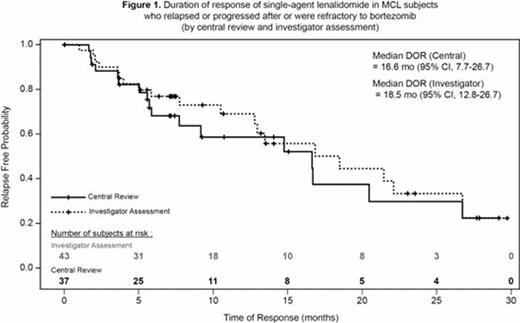
134 subjects with relapsed or refractory MCL who were heavily pretreated (no limitation in number of prior therapies) were enrolled. The median age was 67 y (range, 43–83), two-thirds of them being 65 y or older and 93% with advanced stage disease (stage III-IV). The median number of prior therapies was 4 (range, 2– 10) with 78% of subjects having received ≥ 3 prior lines of treatment. The ORR to single-agent lenalidomide was 28% (CR/CRu 8%; Table 1) by independent central review, with a median DOR of 16.6 mo (95% CI, 7.7–26.7; Figure 1). The ORR was 32% (CR/CRu 16%) by investigator assessment, with a median DOR of 18.5 mo. Median time to response (central review) was 2.2 mo (3.7 mo to achieve CR). The median PFS was 4.0 mo (95% CI, 3.6–5.6); median OS was 19.0 mo (95% CI, 12.5–23.9). Subjects received an average dose of 20 mg/d of lenalidomide. Lenalidomide was dose reduced in 38% of subjects; treatment discontinuation due to an adverse event (AE; primarily myelosuppression) was reported in 19% of subjects. The most common grade 3/4 AEs were neutropenia (43%), thrombocytopenia (28%), anemia (11%), pneumonia (8%) and fatigue (7%). Other AEs (any grade) included tumor flare reaction (13 subjects, 10%), deep vein thrombosis (5 subjects, 4%), pulmonary embolism (3 subjects, 2%) and invasive second primary malignancies (3 subjects, 2%).
The EMERGE study demonstrated rapid and durable efficacy of lenalidomide in MCL subjects who relapsed or progressed after or were refractory to bortezomib. These results in heavily pretreated MCL subjects (median of 4 prior treatments), and with an expected toxicity profile support single-agent lenalidomide in subjects with relapsed or refractory MCL after bortezomib.
Efficacy of single-agent lenalidomide in subjects with MCL relapsed or progressed after or refractory to bortezomib
| Efficacy Parameter (N=134) . | By Central Review (n=134) . | By Investigator Review (n=134) . |
|---|---|---|
| ORR, n (%) | 37 (28) | 43 (32) |
| CR/CRu, n (%) | 10 (8) | 22 (16) |
| PR, n (%) | 27 (20) | 21 (16) |
| Median DOR, mo | 16.6 (95% CI, 7.7–26.7) | 18.5 (95% CI, 12.8–26.7) |
| Median time to response, mo (range) | 2.2 (1.7–13.1) | 2.0 (1.7–15.9) |
| Median TTP, mo | 5.4 (95% CI, 3.7–7.5) | 4.0 (95% CI, 3.6–7.5) |
| Median PFS, mo | 4.0 (95% CI, 3.6–5.6) | 3.8 (95% CI, 3.5–6.8) |
| Efficacy Parameter (N=134) . | By Central Review (n=134) . | By Investigator Review (n=134) . |
|---|---|---|
| ORR, n (%) | 37 (28) | 43 (32) |
| CR/CRu, n (%) | 10 (8) | 22 (16) |
| PR, n (%) | 27 (20) | 21 (16) |
| Median DOR, mo | 16.6 (95% CI, 7.7–26.7) | 18.5 (95% CI, 12.8–26.7) |
| Median time to response, mo (range) | 2.2 (1.7–13.1) | 2.0 (1.7–15.9) |
| Median TTP, mo | 5.4 (95% CI, 3.7–7.5) | 4.0 (95% CI, 3.6–7.5) |
| Median PFS, mo | 4.0 (95% CI, 3.6–5.6) | 3.8 (95% CI, 3.5–6.8) |
CR(u) = complete response (unconfirmed); DOR = duration of response; ORR = overall response rate; PFS = progression-free survival; PR = partial response; TTP = time to progression.
Goy:Pfizer: Advisory board member, Advisory board member Other; Seattle Genetics: Advisory board member Other; J&J: Advisory board member, Advisory board member Other; Pharmacyclics: Advisory board member, Advisory board member Other; Millenium: Advisory board member, Advisory board member Other, Speakers Bureau; Celgene: Advisory board member Other. Off Label Use: This is a phase 2 clinical study of safety and efficacy for lenalidomide in patients with MCL. Sinha:Celgene: Research Funding. Williams:Celgene: Clincial Trial Research Support, Advisory Boards, Data Safety Committee Member, Consultant Other, Consultancy. Drach:Celgene: Speakers Bureau; Janssen: Speakers Bureau; Roche: Research Funding. Ramchandren:Seattle Genetics: Speakers Bureau. Herbrecht:Pfizer: Advisory board member Other. Zhang:Celgene: Employment. Cicero:Celgene: Employment. Fu:Celgene: Employment. Witzig:Celgene : Research Funding.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal