Abstract
Abstract  816
816
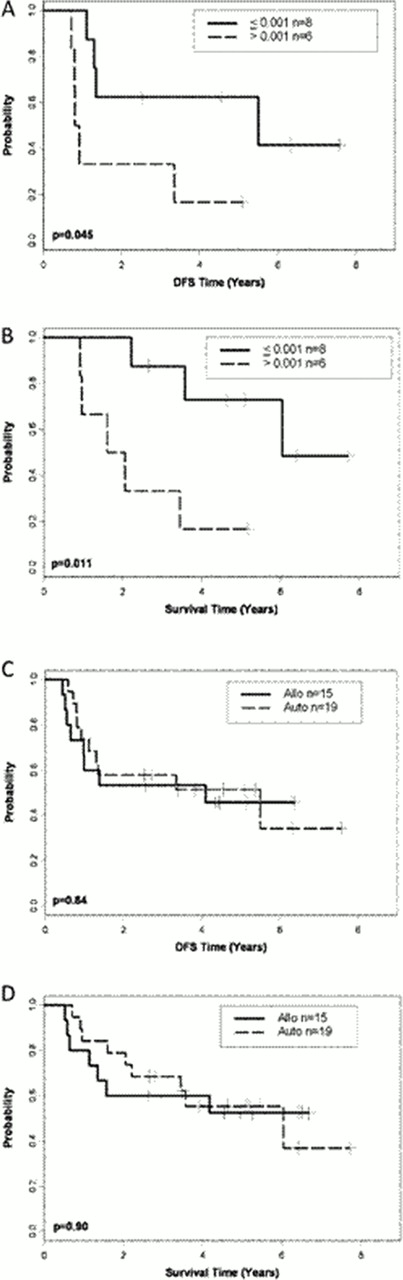
We hypothesized that imatinib plus sequential chemotherapy would result in significant leukemia cell cytoreduction (molecular remission) in patients with Ph+ acute lymphoblastic leukemia (ALL), allowing collection of normal hematopoietic stem cells uncontaminated by residual BCR/ABL+ lymphoblasts and thus reduce the likelihood of relapse after autologous (auto) stem cell transplant (SCT) for patients <60 years old without sibling donors. Cancer and Leukemia Group B (CALGB) study 10001 enrolled 58 patients who received 3–4 courses of imatinib (400 mg twice daily) plus sequential chemotherapy followed either by total body irradiation (TBI, 1320 cGy)/etoposide (60 mg/kg) and an allogeneic (allo) SCT from a matched sibling donor or by TBI/etoposide/cyclophosphamide (100 mg/kg) and auto-SCT. Fifteen had an allo-SCT on study using their matched sibling donor; others were transplanted off study using unrelated donors or received alternative therapy. Nineteen have undergone auto-SCT. Imatinib plus chemotherapy resulted in reverse-transcriptase polymerase chain reaction (RT-PCR) negative stem cells (complete molecular response, CMR) in 9 of the 19 patients; 4 remained minimally positive (major molecular response, MMR; <0.001) and 6 were not evaluable. RT-PCR status of the stem cell products had no effect on overall survival (OS) or disease-free survival (DFS) after auto-SCT (CMR vs. MMR P=0.77 for DFS and P=0.50 for OS). We studied the effect of minimal residual disease (MRD) detection on outcome following auto-SCT. At day +120 post auto-SCT, DFS and OS were longer in patients who achieved at least a MMR (n=8) than patients who did not have a MMR (n=6) at that time point (P=0.045 and P=0.011; Panels A and B). After allo-SCT, 7 of 10 patients who survived >120 days had CMR; 2 additional patients achieved MMR, and 1 patient had residual MRD >0.001 (less than MMR); however, the sample size was too small to analyze the effect of MRD on outcome. After a median follow up time of 5.1 years, 9 (47%) of 19 auto-SCT patients and 7 of 15 (47%) of the allo-SCT patients remain alive in continuous CR. Ten of the transplanted patients have relapsed (8 following auto-SCT and 2 following allo-SCT); relapses occurred at a median of 5.9 months following auto-SCT. The DFS (median, 5.5 vs 4.1 years; P=0.84) and OS (median, 6.0 years vs. not reached at >6 years; P=0.90) were similar between those who underwent auto- or allo-SCT (Panels C and D). We conclude that patients who have MRD at levels lower than or equal to MMR following auto-SCT have prolonged survival and that auto-SCT represents a safe and effective alternative to allo-SCT for Ph+ ALL patients without sibling donors. The intergroup trial CALGB 10701 (Alliance) is now testing this strategy in Ph+ ALL patients >50 year old, using dasatinib as the BCR/ABL inhibitor.
Disease-free (A, C) and overall (B, D) survival, stratified by minimal residual disease (MRD) at Day +120 and by transplant type. (A, B) MRD ≤0.001 (major molecular response) vs. >0.001 at day +120 for patients undergoing autologous-stem cell transplant (SCT); (C, D) allogeneic (allo) vs. autologous (auto) SCT.
Disease-free (A, C) and overall (B, D) survival, stratified by minimal residual disease (MRD) at Day +120 and by transplant type. (A, B) MRD ≤0.001 (major molecular response) vs. >0.001 at day +120 for patients undergoing autologous-stem cell transplant (SCT); (C, D) allogeneic (allo) vs. autologous (auto) SCT.
Wetzler:BMS: Research Funding; Novartis: Research Funding. Off Label Use: Dasatinib is off label but in a clinical trial. 615A Oral Session 1 (3 abstracts) Acute promyelocytic leukemia 622A Oral Session 1 (3 abstracts) Autologous and Allogeneic Transplantatin CLL - Biology and Pathophysiology, excluding Therapy: Cell Signaling Leukemias - Biology, Cytogenetics and Molecular Markers in Diagnosis and Prognosis: Chronic Lymphoid and Myeloid Leukemias 111. Hemoglobinopathies, excluding Thalassemia IV
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal