Abstract
Abstract  655
655
Minimal residual disease (MRD), detectable by multiparametric flow cytometry (MFC) before allogeneic hematopoietic cell transplantation (HCT), is associated with increased relapse and lower disease-free survival (DFS) and overall survival (OS) in acute myeloid leukemia (AML) in first complete remission (CR1). As the relationship between MRD and outcome is less studied for patients in second CR (CR2), we assessed whether this association is similar to that seen in CR1 patients, and examined the impact of MRD levels on outcome.
We studied 253 consecutive patients (median age: 43.1 [range: 0.6–72.6] years) receiving myeloablative HCT for AML in first (n=183) or second (n=70) CR or CR with incomplete blood count recovery (CRi) between May 2006 and November 2011. Pre-HCT bone marrow aspirates were obtained in all patients and analyzed by routine karyotyping and ten-color MFC. MRD was identified as a cell population showing deviation from normal antigen expression patterns as compared with normal or regenerating marrow. Any level of residual disease was considered MRDpos. Data are current as of August 6, 2012.
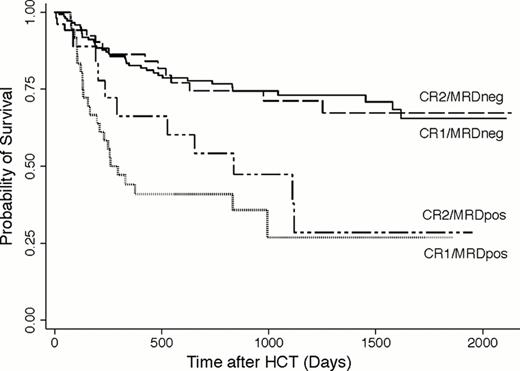
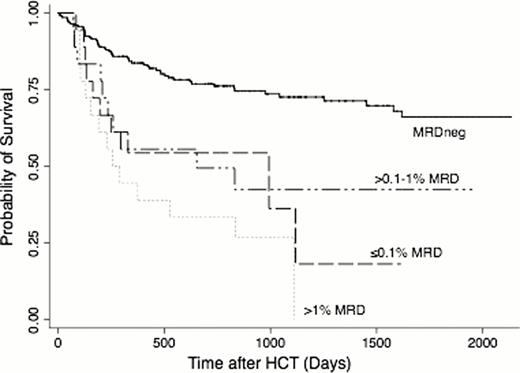
Before HCT, 85.7% of patients met morphological criteria for CR, whereas 14.3% had CRi. Thirty-six (19.7%) patients in CR1 and 18 (25.7%) patients in CR2 had MRD (MRDpos) as determined by MFC, with median of 0.29% (range: 0.007–7.8%) and 0.41% (0.05–3.5%) abnormal blasts for MRDpos CR1 and CR2 patients. Among CR1 patients, the 3-year estimates of OS where 73% (64–80%) and 27% (10–47%) for MRDneg and MRDpos patients, respectively; among CR2 patients, 3-year OS was estimated to be 71% (55–82%) and 47% (23–69%), respectively (Figure 1). Three-year estimates of relapse among CR1 patients were 21% (14–28%) and 59% (41–73%), respectively, and 20% (9–32%) and 68% (37–86%), respectively, among CR2 patients. This relatively similar outcome for CR1 and CR2 patients may be at least partly accounted for by more favorable cytogenetic risk profile of the leukemias in patients transplanted in CR2 (favorable-risk: 22.9% vs. 3.3%; adverse-risk leukemias: 11.4% vs. 26.2%; secondary AML: 8.6% vs. 34.4%). The risk of death for patients who were MRDpos was 3.27-times that among patients who were MRDneg (95% CI, 2.11–5.05; p<0.0001), and the risk of relapse was also increased (hazard ratio [HR]=5.46 [3.39–8.81], p<0.0001). The association of MRD with outcome among patients in CR1 is similar to that among patients in CR2 (p=0.46, p=0.48 tests of interaction for mortality, relapse). If MRD was modeled as a continuous linear variable, both the risk of death and the risk of relapse increased as MRD increased (p=0.001, p<0.0001). Categorizing MRD levels as 0 (n=199), 0.1% or less (n=14), >0.1–1% (n=24), and >1% (n=16), the risk of death increased with each increasing level of MRD relative to the MRDneg group (Figure 2), but the magnitude was fairly similar across all MRDpos groups (HR=2.69, HR=2.96, HR=4.41), and the lowest level of MRD (0.1% or less) was not statistically significantly different from the highest level of MRD (>1%, p=0.30). Similarly, the risks of death in the three MRDpos groups relative to the MRDneg group were HR=5.24, HR=4.32, and HR=8.67, and the difference between the groups with the lowest and highest levels of MRD were not statistically significant (p=0.28). Adjustment for cytogenetic risk, CR duration, pre-HCT karyotype, pre-HCT blood count recovery, and the HCT preparative regimen led to the same qualitative results.
The negative impact of MRD on outcome among AML patients in CR2 is similar to the negative impact seen in patients in CR1. Our data indicate that outcomes of MRDneg AML patients are excellent after myeloablative HCT in either CR1 or CR2. Even patients with minute amounts of MRD (0.1% or less) have significantly worse outcomes than MRDneg patients. Patients with higher levels of MRD (>1%) had worse outcome than those with minute amounts, but the limited number of MRDpos patients limit the power to detect statistically significant differences between these groups. The poor outcome of MRDpos patients provides the rationale for studies investigating whether cure rates could be improved by MRD-directed therapy before, during, or after HCT.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal