Abstract
Abstract 615
The P2Y12 receptor is expressed on the platelet surface and mediates ADP-induced aggregation, but it has recently been shown to be expressed also in other cells of the immune system. Furthermore, the P2Y12 receptor gene variants correlate with pulmonary inflammation and asthma, and P2Y12 receptor deficiency or platelet depletion abrogated dust mite-induced airway inflammation, suggesting an important role for the P2Y12 receptor in pulmonary inflammation. Excessive neutrophil infiltration into the lungs is a hallmark of acute lung injury. The mechanisms that trigger this infiltration are poorly understood but may be the result of the development of a pro-inflammatory phenotype in the lung. Platelets are increasingly recognized for their ability to modulate immune responses through their interaction with neutrophils, dendritic cells, and T lymphocytes. These studies suggest an important regulatory role for the P2Y12 receptor and activated platelets in regulating neutrophil influx into the lung.
Using a mouse model of intra-abdominal sepsis and acute lung injury (cecal ligation and double puncture (CLP)), we examined the role of the P2Y12 receptor in neutrophil migration and lung inflammation using P2Y12 null mice. In this study wild type (WT) and P2Y12 null (KO) mice underwent Sham surgery or CLP surgery (5 animals/group). At 24 hours post surgery, blood and lung tissue samples were collected.
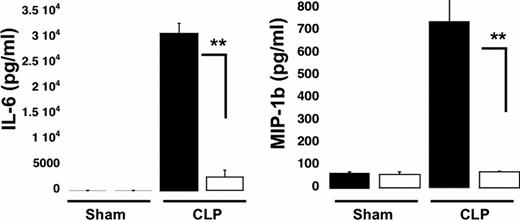
Our results show that the sepsis-induced increase in circulating white blood cells (WBC) was attenuated in CLP KO mice, compared with CLP WT mice (circulating WBC in CLP WT: 1.62 ± 0.41×103cell/ml* compared with CLP KO: 0.8 ± 0.4×103cell/ml, *p<0.05 WT CLP versus KO CLP mice). There were no significant differences in the WBC count in WT Sham surgery animals as compared to the KO sham surgery animals (Sham WT: 0.8 ± 0.2×103cell/ml vs. Sham KO: 0.8 ± 0.1×103cell/ml). Interestingly, no significant changes in the platelet count between the groups were observed. Next we investigated the role of P2Y12 in regulating sepsis-induced elevations in plasma levels of cytokines (TNF-a, IL-10, IL-6 and MIP-1b) (Figure 1). The concentration of each cytokine was elevated during sepsis in both WT and KO animals as compared to sham surgery animals, but, the sepsis-induced increase was significantly lower in P2Y12 null mice (**p < 0.01 KO CLP model versus WT- CLP).
Sepsis-induced increases in plasma cytokines are attenuated in P2Y12 KO mice following CLP. Plasma levels of TNF-a, IL-10, IL-6 and MIP-1b in wild type (black) and KO mice (white). Values are expressed as pg/ml, mean ± SEM (*p < 0.05; **p < 0.01; KO CLP model versus WT CLP, n=5).
Sepsis-induced increases in plasma cytokines are attenuated in P2Y12 KO mice following CLP. Plasma levels of TNF-a, IL-10, IL-6 and MIP-1b in wild type (black) and KO mice (white). Values are expressed as pg/ml, mean ± SEM (*p < 0.05; **p < 0.01; KO CLP model versus WT CLP, n=5).
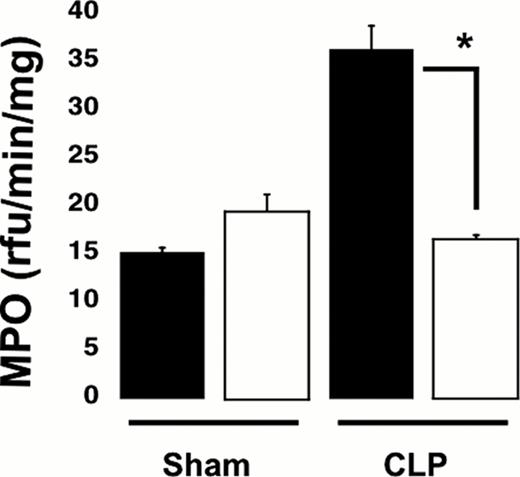
Finally we studied neutrophil infiltration in lung tissues using hematoxylin and eosin (H & E) staining and myeloperoxidase (MPO) activity in lung tissue homogenates. Analysis of H & E staining demonstrated that the increase in inflammatory cell infiltration detected in WT was diminished in KO mice, suggesting a decrease in inflammation in the lungs of P2Y12 null mice. Furthermore, the MPO concentration was significantly decreased in KO CLP lung tissue as compared to WT CLP (Figure 2), (*p < 0.05; KO CLP model versus wild type).
Sepsis-induced elevations in lung MPO levels are reduced in P2Y12 null mice. MPO analysis was performed in lung samples of Sham and CLP in wild type (black) and KO mice (white). Values are expressed as rfu/min/mg, mean ± SEM (*p < 0.05; KO CLP model versus WT, n=5).
Sepsis-induced elevations in lung MPO levels are reduced in P2Y12 null mice. MPO analysis was performed in lung samples of Sham and CLP in wild type (black) and KO mice (white). Values are expressed as rfu/min/mg, mean ± SEM (*p < 0.05; KO CLP model versus WT, n=5).
In conclusion, our data shows a decrease in plasma cytokine levels and in circulating WBC, as well as a diminished neutrophil infiltration in the lungs indicating that P2Y12 null mice are protected against sepsis-induced lung injury. This suggests a regulatory role for P2Y12 receptor during inflammation.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal