Abstract
Abstract 4807
The role of the 2B4/CD48 pathway in chronic viral infections has been controversial, with some studies suggesting an enhancing and others an inhibitory effect on virus specific CD8 (+) cytotoxic T-lymphocytes. Human T-cell lymphotropic virus type 1 (HTLV-1) trans-activating (Tax) protein is crucial for viral replication and activates HTLV-1 gene transcription.
Peripheral blood mononuclear cells (PBMCs) were isolated by Ficoll centrifugation and cryopreserved until use. For staining, cells were incubated with fluorochrome-conjugated antibodies against 2B4, CD48, CD4, CD8. CD8 (+) lymphocytes were identified using phycoerythrin (PE) labeled tetramers HLA*0201 or 2402 Tax, CMV and EBV immune-dominant epitopes. In some, cells were fixed and permeabilized prior to staining with FITC-conjugated anti-Tax protein (clone Lt-4) antibodies or a control IgG. To examine the effect of CD48 blockade on Cd8+ T lymphocyte function, PBMCs (1 × 106) were cultured in complete medium (RPMI-1640 supplemented with 100U/ml penicillin, 0.1mg/ml streptomycin, 0.05mM 2-mercaptoethanol, 50U/ml recombinant interleukin-2 and 10% inactivated FCS) with/without Tax peptide and/or anti-CD48 blocking antibody (5μg/ml) in the presence of brefeldin or monensin for intracellular perforin or surface degranulation of CD107a, respectively. Acquisition was done using FACS Calibur or FACS Scan flow-cytometer and analyzed with Flowjo software. Differences were analyzed by Mann-Whitney U test; changes pre- and post- ex-vivo culture were analyzed by Wilcoxon matched pairs test using Stata software version 9 (StataCorp LP). P-values <0.05 were considered significant.
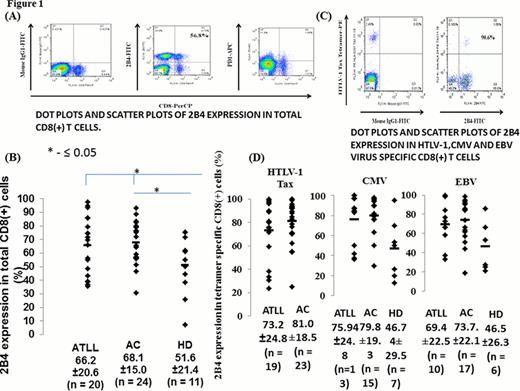
The expression of 2B4 in total CD8 (+) T-cells was significantly greater in HTLV-1 infected individuals compared to healthy controls(67.2±17.6 and 51.6±21.4, respectively; p 0.03; n = 11 HCs, 44 infected; Figure 1b), there was no difference in 2B4 expression in HTLV-1 tetramer specific CD8 (+) CTLs between asymptomatic HTLV-1 infected (ACs) and ATLL (81.0±18.5 and 73.2±24.8, respectively; n = 23 ACs, 19 ATLLs; Figure 1d) individuals.
In CMV specific CTLs, 2B4 expression was significantly higher in HTLV-1 infected individuals compared with uninfected healthy controls (77.4±21.8 and 46.7±29.5, respectively; p = 0.01, n = 28 infected and 7 HCs; Figure 1D); and 2B4 expression was also greater in EBV-specific CTLs in HTLV-1 infected individuals compared with uninfected healthy controls (72.7±22.1 and 46.5±26.3, respectively; p = 0.02; n = 27 infected and 6 HCs; Figure 1d).
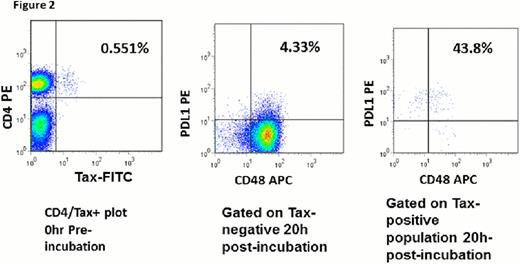
HTLV-1 infected cells express intracellular HTLV-1 Tax protein in ex vivo cultures, peaking at 16 to 18 hours followed by a marked fall to undetectable levels. In our study, we noticed a trend whereby CD48 expression was lower in intracellular Tax-protein expressing (infected) CD4 (+) cells compared to Tax protein-negative (uninfected cells) in short term ex vivo cultures (50.6±7.3% versus 90.1±5.6%, respectively); and CD48 expression in total CD4 (+) cells was downregulated as Tax protein became expressed in cultures (93.3±3.3 at start versus 86.7±6.1 after culture; 3 independent experiments; Figure 2). Programmed death ligand 1 (PDL1) is known to be upregulated in Tax-protein expressing compared with Tax-negative cells, which is believed to permit immune evasion by HTLV-1 infected cells, but the proportion of HTLV-1 infected cells that are CD48(+) appears to be down regulated in this study. In vitro infection of target cells with EBV has been shown to upregulate CD48 expression at the mRNA and protein levels; thus, indicating that downregulation of CD48 in HTLV-1 infected cells is unique, possibly related to interactions of the trans-activating viral protein with host gene regulatory mechanisms at molecular level.
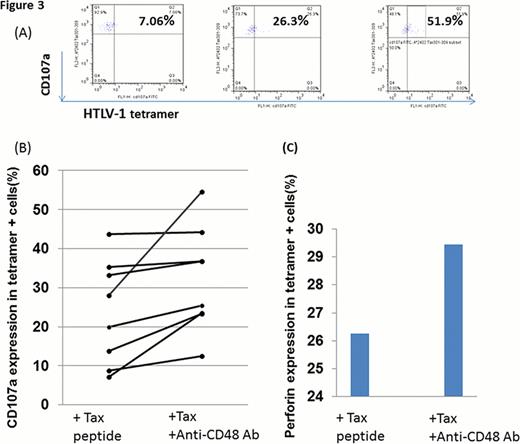
Further, perforin production by CD8(+) lymphocytes is known to mediate lysis of HTLV-1 infected cells, in our study, the CD48 antibody resulted in increased perforin production by CD8 (+) CTLs, suggesting an inhibitory role for the 2B4/CD48 pathway in HTLV-1 infection(Figure 3c).
Similarly, expression of the degranulation marker, CD107a in CD8 (+) lymphocytes was augmented with CD48 blocking antibody (Figure 3a, b).
The pattern of 2B4 expression in HTLV-1 infection and enhanced CD8 (+) lymphocyte function with 2B4/CD48 blockade suggest involvement of this pathway in immune exhaustion, and a possible immunotherapeutic target.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal