Abstract
Abstract 47
In adults with newly diagnosed, poor-risk AML we previously demonstrated that induction therapy with Flavopiridol in combination with Ara-C and Mitoxantrone (FLAM,) in a timed-sequential manner, yields complete remission (CR) rates of 67% and median disease-free survival (DFS) of 13.6 months. These data suggest that FLAM might improve outcomes relative to standard “7+3” in newly diagnosed AML with poor-risk features. Thus, we are conducting a phase II randomized trial comparing the CR rates with FLAM vs. 7+3 induction for all newly diagnosed adult patients with AML with intermediate and poor-risk features. We also previously identified a population of CD34+CD38− cells with intermediate aldehyde dehydrogenase (ALDH) activity that appears to represent a leukemic stem cell (LSC) population. We are examining the effects of FLAM vs. 7+3 in eradicating this LSC population and determining if persistence of LSC's predicts for relapse.
The primary objective is to compare the rate of CR after 1 cycle of FLAM vs. 1 cycle of 7+3. The secondary objectives are to compare toxicities, survival rates, and presence or absence of minimal residual disease (MRD) after both induction regimens.
All newly diagnosed adult patients aged 18–70 without favorable risk features were randomized in a 2:1 fashion between FLAM and 7+3 at 90 mg/m2 of Daunorubicin. To achieve balanced randomization, patients were stratified by age, secondary AML and leukocyte count. CD34+ cell subsets were analyzed by flow cytometry for CD38 expression and ALDH activity by Aldefluor. The trial is ongoing.
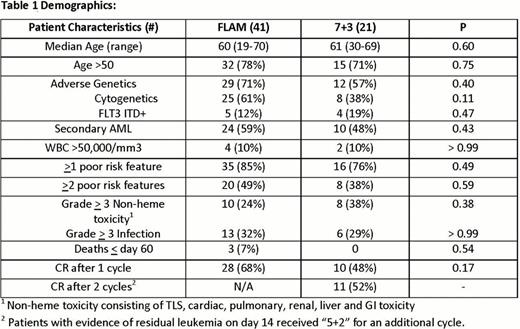
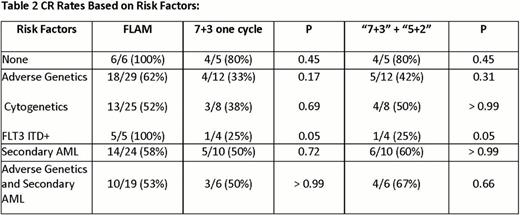
To date, 62 patients are evaluable for the primary end point. Demographics and results are depicted in table 1. Patient characteristics were similar in both arms, although there were more patients with poor risk features in the FLAM arm than 7+3. Overall grade > 3 toxicity was similar between both arms. However, there were 3 cases of grade > 3 tumor lysis syndrome (TLS) with FLAM, with 1 death, vs. 0 with 7+3. There were also 3 deaths with FLAM before day 60 vs. 0 with 7+3. CR rate with FLAM was 68% (28/41), with 1 cycle of 7+3 was 48% (10/21) (p = 0.17), and with 2 cycles (7+3 + 5+2) was 52% (11/21). CR rates based on specific risk factors are depicted in table 2. There was a trend toward improved CR rates in patients with adverse genetics with FLAM (p = 0.17). Putative AML LSC's were distinguished from normal hematopoietic stem cells based on ALDH activity. The persistence of putative LSC's in patients after therapy was highly predictive of subsequent clinical relapse.
Based on the current CR rate of 68%, FLAM holds promise as a therapeutic option for patients with newly diagnosed AML with intermediate and poor risk features. In this preliminary analysis, there is a suggestion that FLAM leads to higher CR rates in patients with adverse genetics than 7+3. Additionally, the detection of MRD consisting of a residual putative LSC population strongly portended subsequent relapse, even in patients without evidence of leukemia.
Off Label Use: Flavopiridol (Alvocidib) is an anti-leukemia agent.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal