Abstract
Abstract  424
424
Azacitidine for injection has been shown to prolong overall survival in patients (pts) with higher-risk myelodysplastic syndromes (MDS) compared with conventional care regimens (CCR) (Lancet Oncol, 2009). An oral formulation of azacitidine (CC-486) is in development. Oral azacitidine may maximize convenience, eliminate injection-site reactions, and if administered in extended dosing schedules, may enhance and prolong the therapeutic effects of azacitidine. Oral azacitidine administered once-daily (QD) for 7 days (d) of repeated 28d cycles has been shown to be bioavailable, biologically and clinically active, and well-tolerated in pts with MDS and acute myeloid leukemia (Garcia-Manero, J Clin Oncol, 2011). Preliminary evidence suggests that extending oral azacitidine dosing to 14d or 21d of the 28d cycle may enhance pharmacodynamic and epigenetic activity (Laille, Leuk Res, 2011).
To evaluate hematologic response and safety associated with extended dosing regimens of oral azacitidine in pts with lower-risk MDS.
This ongoing, multicenter, phase 1 study, enrolled pts with lower-risk (IPSS Low or INT-1) MDS who were RBC transfusion dependent (TD) and/or thrombocytopenic (average platelet count ≤50,000 within 56d prior to the first dose) at baseline. Pts were sequentially assigned to receive oral azacitidine 300mg QD for either 14d or 21d of repeated 28d cycles. Hematologic assessments were made every 2 weeks. Hematologic response was assessed using IWG 2006 criteria (Cheson, Blood, 2006). Adverse events (AEs) were graded using NCI-CTCAE version 3.0.
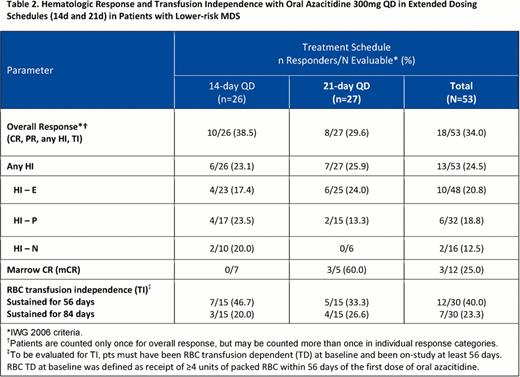
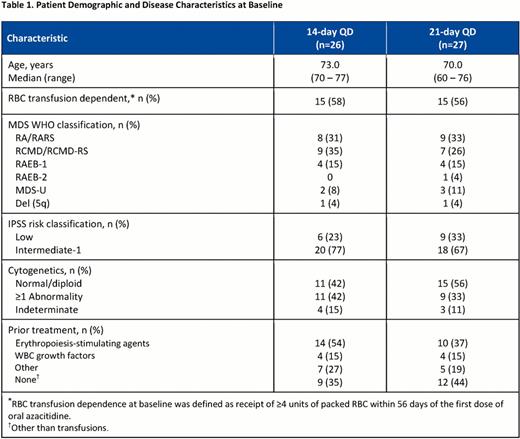
At data cut-off (May 18, 2012), 53 pts with lower-risk MDS had enrolled (300mg oral azacitidine QDx14d, n=26; QDx21d, n=27). Demographic and disease characteristics at baseline were similar in the 14d and 21d treatment cohorts (Table 1 ). Median (range) hematology counts at baseline were Hgb 8.7 g/L (6.0–13.0), ANC 1.6×109/L (0–30.3), and platelets 56.0×109/L (6.0–564.0). At study entry, 40% of pts had received no prior MDS treatment (except transfusions), 45% had received erythropoiesis-stimulating agents, and 15% had received WBC growth factors. The number of oral azacitidine treatment cycles received ranged from 1 to 12 (median numbers of oral azacitidine cycles were 6 in the QDx14d and 4 in the QDx21d cohorts). Four pts in the 21d cohort and 1 pt in the 14d cohort received reduced oral azacitidine doses (200mg QD). Overall, 10 pts discontinued the study, including 6 pts (3 pts in each cohort) who discontinued due to AEs that may have been treatment-related (gastrointestinal [n=2] or intracranial [n=1] hemorrhage, febrile neutropenia [n=1], pneumonia [n=1], thrombocytopenia [n=1]). Overall response rates (ORR), which included complete (CR) and partial remission (PR), any hematologic improvement (HI), and transfusion independence (TI), ranged from 38.5% in the QDx14d cohort to 29.6% in the QDx21d cohort, and RBC TI was achieved by 47% and 33%, respectively, of pts who were RBC TD at baseline (Table 2 ). For pts who received at least 4 cycles of oral azacitidine (14d, n=19; 21d, n=14), ORR was 47.4% in the 14d and 50.0% in the 21d cohorts, and RBC TI rates in RBC TD pts (n=16) were 67% in the 14d and 57% in the 21d cohorts. The most frequent (≥5%) grade 3/4 hematologic AEs in the QDx14d cohort were anemia (11.5%), thrombocytopenia (11.5%), and neutropenia (7.7%); and in the QDx21d cohort were neutropenia (14.8%), anemia (7.4%), and febrile neutropenia (7.4%). Most frequent grade 3/4 non-hematologic AEs were gastrointestinal, including vomiting (7.7%) in the QDx14d cohort, and diarrhea (11.1%) and vomiting (7.4%) in the QDx21d cohort.
Oral azacitidine 300mg QD administered in extended dosing schedules of 14d or 21d of repeated 28d cycles was effective and well-tolerated in these pts with lower-risk MDS. Beside hematologic AEs, the most frequently observed AEs with oral azacitidine were gastrointestinal and were manageable. Efficacy and safety outcomes with 300mg QD oral azacitidine were generally comparable between the 14d and 21d extended dosing regimens. Based on these data, oral azacitidine administered once-daily in extended dosing schedules is active and well-tolerated and warrants further investigation in randomized, controlled trials.
Hematologic Response and Transfusion Independence with Oral Azacitidine 300mg QD in Extended Dosing Schedules (124 and 21d) in Patients with Lower-risk MDS
Garcia-Manero:Celgene: Research Funding, Speakers Bureau. Gore:Celgene Corporation: Consultancy, Research Funding. Scott:Celgene Corporation: Honoraria, Research Funding, Speakers Bureau. Hetzer:Celgene Corporation: Employment, Equity Ownership. Kumar:Celgene Corporation: Employment, Equity Ownership. Skikne:Celgene: Employment, Equity Ownership.
Author notes
Asterisk with author names denotes non-ASH members.

This icon denotes a clinically relevant abstract

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal