Abstract
Abstract 4052
In patient (pts) with advanced MM, clinical parameters such as low platelet count and high serum creatinine levels carry poor prognosis (Kumar SK, et al. Mayo Clin Proc. 2004;79:867-74). At this disease stage, outcome of MM therapy should be assessed not only with the traditional parameters (progression-free survival, duration of response, and overall survival), but also by evaluating meaningful clinical parameters. POM a novel immunomodulatory agent, in combination with LoDEX, has demonstrated encouraging clinical activity and favorable tolerability in pts with RRMM in the multicenter, randomized, open-label MM-002 phase 1/2 study (Richardson PG, et al. Blood 2011;118:abs 634). This study evaluated changes in clinically important disease parameters with potential prognostic significance; data from the cut-off date of 30 March 2012 are presented.
Pts with MM and ≥2 prior therapies including LEN and BORT, and disease progression during or within 60 days of their last treatment, were randomized to POM+LoDEX (POM, 4 mg/day for days 1–21 of a 28-day cycle (C); LoDEX, 40 mg/week) or POM alone. At progression, patients receiving Pom alone could receive POM+LoDEX at the discretion of the investigatior. All pts received mandatory thromboprophylaxis (daily low-dose aspirin). We herein present the effect of POM+LoDEX on end-organ functional parameters, including platelet count, serum calcium, serum creatinine (SCr), albumin (Alb), residual nonaffected normal immunoglobulins (Ig) (other than M-protein), hemoglobin (Hgb), and ECOG performance status. Improvement from baseline parameters was assessed by evaluating the change in end-organ functional parameters from pre-therapy to best post-therapy levels. Improvement was defined as a shift from abnormal to normal, with a normal platelet count defined as 150–350 × 103/mm3; calcium level 8.3–10.6 mg/dL; SCr level 0.9–1.5 mg/dL; increase of serum Alb ≥0.5 g/dL; Ig levels: IgA >70 mg/dL, IgG >565 mg/dL, and IgM >40 mg/dL; Hgb increase ≥1 g/dL; and decrease in ECOG performance status of at least 1 score. No allowance was made for the possible confounding influence of the use of blood or platelet transfusions.
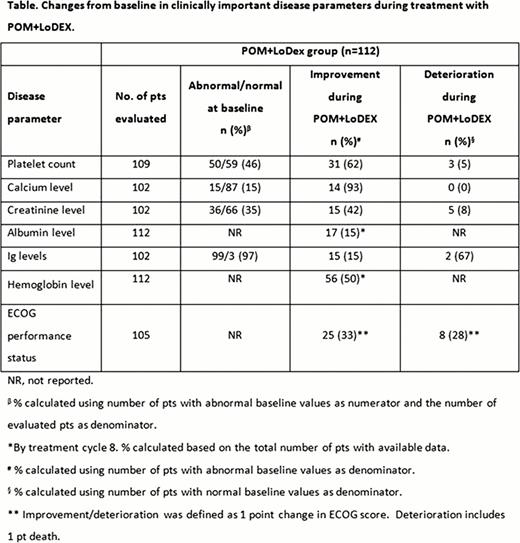
Of the 113 pts who were randomized to receive POM+LoDex, 112 pts were evaluable for safety. Median number of prior therapies was 5, median treatment duration with POM+LoDEX was 5.0 months, and median number of cycles was 5. A substantial number of pts had abnormal baseline platelet count, calcium, SCr, and Ig levels (Table). During POM+LoDEX treatment, platelet counts, calcium, and SCr levels improved in 62%, 93%, and 42% of pts with abnormal values at baseline, respectively. Most pts treated with POM+LoDEX (97%) had low IgA, IgG, or IgM at baseline. Of those pts, Ig levels improved into a normal range in 15% of pts. Hgb levels increased by ≥1 g/dL in 30% of pts by the end of C2, 41% by C4, and 50% by C8. Alb levels increased by ≥0.5 g/dL in 5% of pts by C2, 8% by C4, and 15% by C8. At baseline, 29 pts in the POM+LoDEX group had an ECOG score of 0, 66 pts had a score of 1 and 10 pts had a score of 2. During treatment, 2 of the 10 pts with a score of 2 improved to 1 and 1 improved to a score of 0; 6 pts maintained their baseline score of 2, 1 pt deteriorated from score 2 to 4. Of those with a baseline score of 1, 22 pts improved to a score of 0, 41 maintained their score of 1 and 3 pts deteriorated (2 to a score of 2 and 1 to a score of 5). Twenty-three pts maintained their ECOG score of 0; 6 pts deteriorated to a score of 1. Overall, there was no incidence of grade 3 or 4 peripheral neuropathy and there was a low overall incidence of hypercoagulable events 4% (2% DVT and 2% PE). Therefore disease parameters associated with these adverse events were not assessed.
Treatment with POM+LoDEX is associated with an improvement in clinically important disease parameters including, platelet count, serum calcium, SCr, Alb, Ig, and Hgb. ECOG status also normalized or improved in 33% of pts. Improvements in end-organ functional parameters may improve the prognosis and facilitate clinical benefit for advanced RRMM pts, with the ability to continue effective therapy enhanced accordingly – an observation which warrants further clinical study, both comparatively and in combination with other active agents in this setting.
Changes from baseline in clinically important disease parameter during treatment with POM+LoDEX.
Lonial:Millennium, Celgene, Novartis, BMS, Onyx, Merck; all < $10,000 per year and disclosed to my institution: Consultancy. Off Label Use: Pomalidomide is an investigational drug and is not approved for the treatment of patients with any condition. Baz:Celgene, Millennium, Bristol Myers Squibb, Novartis: Research Funding. Bahlis:Celgene: Honoraria; Johnson and Johnson: Honoraria, Research Funding. Chen:Johnson & Johnson, Celgene, GlaxoSmithKline: Research Funding; Johnson & Johnson, Lundbeck, Celgene: Consultancy; Roche: Honoraria. Anderson:Acetylon, Oncopep: Scientific Founder, Scientific Founder Other; Celgene, Millennium, BMS, Onyx: Membership on an entity's Board of Directors or advisory committees. Chen:Celgene: Employment, Equity Ownership. Zaki:Celgene: Employment, Equity Ownership. Richardson:Celgene, Millennium, Johnson & Johnson: Advisory Board Other.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal