Abstract
Abstract 3889
Inflammatory cytokines play a biological role in the pathogenesis of Chronic lymphocytic leukemia (CLL). IL23 is a pro-inflammatory cytokine involved in T-cell responses and in tissue remodeling. It has been shown that the IL23 receptor (IL23R) is up-regulated in primary acute lymphoblastic leukemia (ALL) cells, and that IL23 inhibits ALL cell growth. Nevertheless, the anti-tumor function of IL23 still remains controversial. The role of the IL23R/IL23 axis in CLL has not been investigated so far. Herein we evaluated the expression pattern of IL23R/IL23 axis and its correlation with progression free survival (PFS) in CLL patients.
A total of 233 newly diagnosed Binet stage A CLL cases from Italian institutions (clinicaltrials.gov NCT00917540) were studied for IL23R expression by flow-cytometry (FC) (median percentage IL23R expression=22.7, range 1.2–91.1). The median follow-up was 23 months (range 1–47). PFS information was obtained in 203 patients. Using the median value of 23% of IL23R as threshold, 8/102 IL23Rneg and 23/101 IL23Rpos CLL cases progressed with therapy requirement. The 2-year PFS probability of IL23Rneg patients was 89.7% as compared to 80.7% of IL23Rpos cases [χ2 7.7, P=.006; HR=3.0, 95%CI (1.3–6.6)]. Cases were then stratified according to IL23R positivity [IL23Rneg (102 cases) versus IL23Rpos (101 cases)]. No significant difference in terms of CD38 and ZAP-70 positive cases was observed, however, the IGVH mutational status could distinguish the two groups: IGHV-mutated in 92 (78.6%) of IL23Rneg vs 70 (61.9%) IL23Rpos and IGHV-unmutated in 25 (21.4%) vs 43 (38.1%), p=.006]. FISH analysis showed that IL23Rneg and IL23Rpos cases carrying 13q14.3 were respectively 53 (51.4%) and 44 (42.7%), while the number of patients with trisomy 12 were 8 and 10 respectively in cases with low and high IL23R expression. Deletion of 11q was detected in 3.9% (4/103) of IL23Rneg and in 8.7% (9/103) of IL23Rpos cases. Only 3 cases with 17p deletion were seen in this cohort of early CLL patients and all belonged to the IL23Rpos group. Overall, no significant differences in the incidence of the major genetic lesions were observed between the two groups. Il23R expression still remained independently associated with PFS also in multivariate analysis.
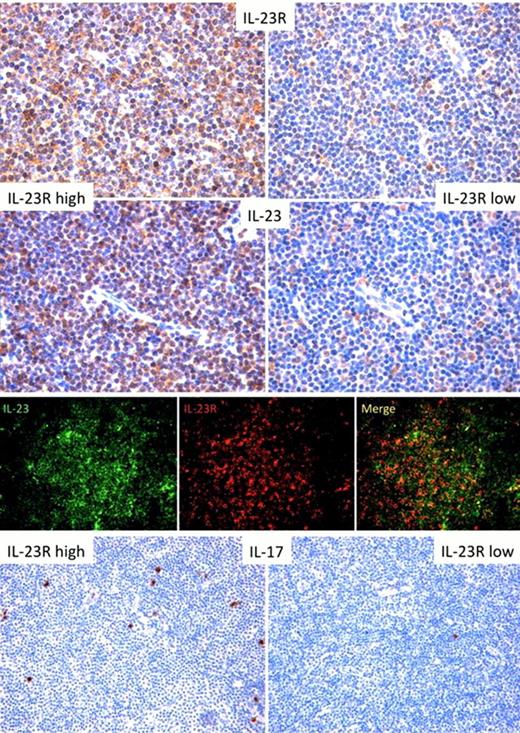
In situ expression analysis of IL23R and of its ligand IL23 was then performed by immunohistochemistry (IHC) in 16 CLL samples [10 lymph node (LN) and 6 bone marrow (BM) biopsies] collected on diagnosis and in 8 control biopsies (4 lymph nodes with reactive follicular hyperplasia and 4 normal BM biopsies). IL23R was variably expressed in CLL and significantly expressed in the neoplastic clones of 9 (6 lymph nodes and 3 BM biopsies) of the 16 cases tested; IL23R was diffusely present along the membrane and cytoplasm of neoplastic cells effacing the lymph node or BM architecture (Fig. 1, upper-left). In CLL cases with low IL23R expression, IL23R was detected in few scattered lymphoid cells intermingling with neoplastic lymphocytes (Fig. 1, upper-right). IL23 was also detected, with a variable staining intensity (Fig. 1, middle-left), paralleling in part that of IL23R. Double-marker analysis confirmed the concomitant expression of IL23 and IL23R in CLL neoplastic infiltrates highlighting the co-localization of the two markers (Fig.1 middle-right) and suggesting the possibility of an autocrine IL23/IL23R loop in CLL clones.
We speculated that the microenvironment of CLL cases rich in IL23R and IL23 could be enriched in IL17-producing cells. The IHC expression of IL17 in CLL cases with low or high IL23R and IL23 expression showed that CLL cases rich in IL23Rpos cells, also characterized by high IL23 expression, displayed significantly higher numbers of IL17pos infiltrating cells (Fig. 1 bottom-left), as compared with CLL cases with no or low expression of IL23R or IL23 (Fig. 1 bottom-right).
In conclusion, our study shows that high IL23R expression predicts a worse PFS. Furthermore, we linked this picture with, the in situ engendering of a clone-related microenvironment characterized by the preponderancy of pro-inflammatory signals such as those of the IL23/IL23R/IL17 axis, and its correlates in the peripheral blood (i.e. IL23R expression on circulating CLL cells), may endorse its strong prognostic significance. This analysis prompts further investigation into the specific function of the IL23/IL23R/IL17 axis and its targets in the context of CLL.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal