Abstract
Abstract 3326
Immune thrombocytopenia (ITP) is a relatively prevalent disease in dogs with significant morbidity and mortality. Canine ITP is clinically analogous to human ITP, with heterogeneity in bleeding manifestations in individuals with similar platelet counts. With a view to ultimately investigate this bleeding heterogeneity, we set out to develop a canine model of ITP. There are currently no existing large animal models of ITP. An induced canine ITP model would be representative of ITP without the confounding co-morbidities seen in clinical cases. Since spontaneous ITP occurs in both dogs and humans, the dog is an ideal translational model.
We hypothesized that 2F9, a murine IgG2a monoclonal antibody to the canine platelet glycoprotein GPIIb (a common target of autoantibodies in ITP), would induce predictable dose-dependent thrombocytopenia (TCP) in healthy dogs. 2F9 had not been previously administered in vivo.
We produced highly purified 2F9 and αYFA antibodies from the 2F9 hybridoma (gift of David Wilcox, Blood Research Institute, Wisconsin) and an isotype control murine anti-yellow fever antibody (αYFA) hybridoma. A dose titration (2 dogs) and a dose repeatability study (3 dogs) were performed in healthy adult research dogs by repeated intravenous infusion (≤ 6 doses) of 2F9 antibody until a target nadir of 5–30 × 103 platelets/μl was reached. Platelet counts were performed hourly until the platelet count reached the desired nadir range (t=0 hrs), after which complete blood counts were performed at 2, 4, 6, 8, 12, 24 hours, then q 24 hours for 10 days. The following were evaluated throughout the study: physical examination, buccal mucosal bleeding time (BMBT, baseline and t=0 only), serum cytokines and chemokines (INFγ, Interleukin (IL) 2, 6, 7, 8, 10, 15, 18, KC, IP-10, MCP-1, GM-CSF, TNFα; Milliplex CCYTOMAG-90K), fibrinogen, and D-dimers.
Specificity of the 2F9 effect was confirmed by IV infusion of the isotype control (αYFA) to 3 dogs at the highest cumulative effective dose of 2F9 (167 μg/kg); all parameters were measured as above (t=0 hrs was one hour after αYFA dosing).
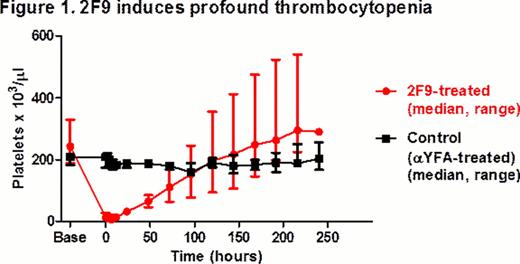
Within 2 hours of a median cumulative 2F9 administration of 63 μg/kg (range 50.0–166.6 μg/kg), all dogs developed profound TCP (range 11–28 × 103/μl). Compared to the control group, platelet nadir was significantly lower (median (range): 6 (4–11) × 103/μl vs. 200 (179–209) × 103/μl; p= 0.036) and change in platelet count from baseline to nadir was significantly greater in the 2F9-treated group (median (range): 238 (179–325) × 103/μl vs. 4 (0–10) × 103/μl; p=0.036) (Fig 1); p-values were calculated using the exact Wilcoxon rank-sum test. Platelet nadir was in our target range and platelet count remained < 40 × 103/μl in all 2F9-treated dogs for 24 hours. Dosing was predictable: in each dog, after an initial dose of 50 μg/kg 2F9, the second dose needed to reach the target nadir could be accurately calculated from the initial platelet decrease.
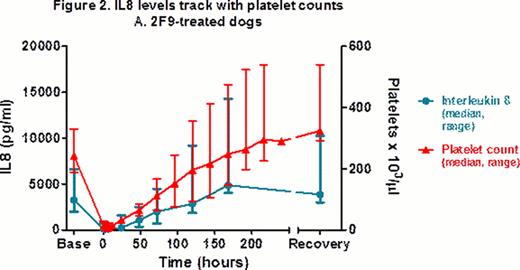
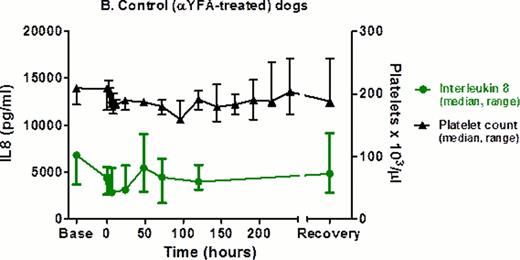
2F9-treated dogs developed a range of clinical bleeding from none to petechiae, ecchymoses, melena, and hematuria. At t=0 hrs, BMBT increased 3–8 fold in treated dogs, compared to < 2 fold in control dogs. Dogs had no changes in vital signs or demeanor and did not require any transfusion support. The model does not appear pro-thrombotic as fibrinogen and D-dimers were similar over time in 2F9-treated vs. control dogs. 2F9 infusion also generated negligible systemic inflammation, as assessed by white blood cell count and serum cytokine measurement. Unexpectedly, however, serum IL8 tracked faithfully with platelet count, demonstrating that platelets are a major source of serum IL8 in dogs (Fig 2). Although α granules are known to contain IL8, platelets have not been previously described as a significant serum IL8 source. Since IL8 is an important neutrophil chemokine, our finding may illuminate a novel mechanism of platelet-neutrophil cross-talk.
In summary, we have developed a novel large animal ITP model that is highly representative of the spontaneous disease. Like naturally-occurring ITP, dogs demonstrate bleeding heterogeneity despite similar platelet counts (data not shown). We expect our model to lead to further insights into bleeding mechanisms in ITP. Ultimately, understanding what factors predispose certain patients to bleed will allow us to exploit these factors therapeutically as novel ITP treatments.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal