Abstract
Therapeutic options for patients with relapsed multiple myeloma (MM) after autologous stem cell transplantation (ASCT) include novel agents, conventional chemotherapy or salvage ASCT, with no standard of care.
We retrospectively reviewed all myeloma patients who relapsed after upfront ASCT and received an autologous re-transplantation as salvage therapy at our center over a period of 15 years (n=200). The aim of our study was to evaluate the role of salvage ASCT in terms of efficacy and to define patients who may benefit most from this treatment approach.
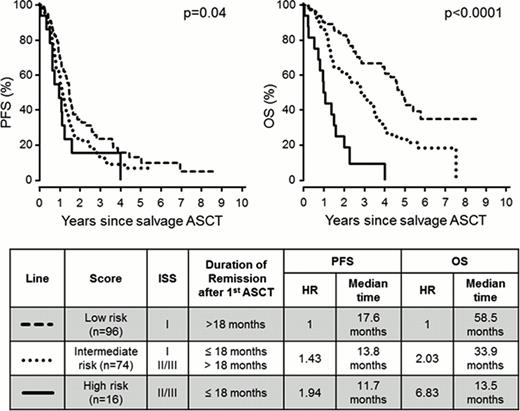
The median follow up after salvage ASCT was 57.1 months. Median progression-free survival (PFS) and overall survival (OS) after salvage ASCT were 15.2 and 42.3 months, respectively. Overall response rate (≥partial response) was 80.4% at day 100, excluding 6 patients who died before assessment. Factors associated with improved PFS and OS after salvage ASCT included remission duration of >18 months after upfront ASCT, bortezomib- or lenalidomide containing therapies for reinduction, response to reinduction, ISS stage I prior to salvage ASCT and year of salvage ASCT 2005 or thereafter. The remission duration after upfront ASCT and the ISS prior to salvage ASCT were combined to create a risk-stratification model with three distinct prognostic groups.
Salvage ASCT causes low morbidity and is capable of achieving sustained disease control in patients with myeloma. The use of lenalidomide and bortezomib for reinduction has improved the results after salvage ASCT, suggesting that novel agents and salvage ASCT are complementary rather than alternative treatment approaches. Stratification by duration of remission after upfront ASCT and ISS allows prediction of survival after salvage ASCT. To circumvent the problem of stem cell harvest in intensively pretreated patients, we recommend collecting and cryopreserving sufficient stem cells for both upfront and salvage ASCT early in the course of myeloma treatment.
No relevant conflicts of interest to declare.

This icon denotes a clinically relevant abstract
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal