Abstract
Abstract 3048
In solid organ transplantation, lifelong immunosuppression is required to preserve graft function, and medication nonadherence is a major risk factor for graft failure. Tacrolimus (Tac) was first developed as an oral twice-daily formulation (Tac BID) and has been widely used in hematopoietic stem cell and solid transplantation, but long-term adherence remains a concern. In renal transplant patients, morning dosing is associated with significantly higher adherence than evening dosing. In response to this potential adherence problem, a once-daily modified release formulation (Tac QD) has been developed with a morning dosing regimen that maximizes the potential for adherence. However, several investigators have recently reported a sustained decrease in Tac exposure in kidney transplant recipients after conversion from Tac BID to Tac QD. In this study, we measured Tac exposure after switching from Tac intravenous infusion (Tac iv) to Tac QD and analyzed the pharmacokinetics (PK) of Tac QD to investigate the correlation between area-under-curve (AUC) and trough in patients who received allogeneic hematopoietic stem cell transplantation (HSCT). This is the first report of the PK study of Tac QD in patients who received allogeneic HSCT.
Patients who were 15–65 years of age and received HSCT from unrelated donors were eligible. They received Tac iv 0.03mg/kg by continuous infusion beginning one day before transplantation, and administration was converted to Tac QD at a 1:4 ratio when the patients engrafted and could tolerate oral medication. Doses were modified to maintain whole-blood trough concentration of 8–12 ng/ml. To analyze PK of Tac QD, plasma samples were obtained at baseline and at 0, 1, 2, 3, 6, 12, and 24 hours after the once-daily administration. Plasma concentration of Tac was determined by an ELISA method.
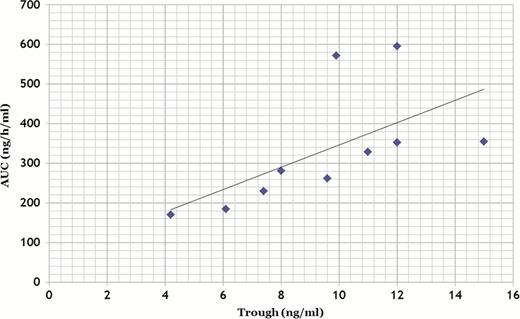
A total of 10 patients with hematological malignancies (AML 6, ALL 1, MDS 2, NHL 1) were enrolled in the PK study. Median age was 45 (23–65) years. Five patients received myeloablative preparative regimens, and 5 patients received reduced intensity regimens, and stem cell sources were bone marrow (BM) from HLA-matched unrelated donor (n=4), BM from HLA DRB1 mismatched unrelated donor (n=4), or cord blood (n=2). After conversion from Tac iv to Tac QD, six out of 10 patients (60%) showed a sustained decrease in Tac exposure and required an increase in their Tac QD daily dose. One patient experienced a quick decrease of more than 78% in trough level of Tac QD, and he developed grade II acute GVHD after the conversion. No other patients developed grade II-IV acute GVHD. None of the patients had to discontinue the agent because of adverse effects, and none developed treatment-related mortality within 100 days after HSCT. In PK analysis, median area-under-curve (AUC) was 246 ng·h/ml. There was a strong correlation between AUC and trough level (Figure 1). Obtaining over 240 ng•h/ml of AUC required 7.5 ng/ml of whole-blood trough levels of tacrolimus.
Despite initial reports showing the bioequivalence of Tac QD with Tac BID, we found that 60% of patients experienced a sustained decrease in Tac exposure. Therefore, the conversion from Tac iv to Tac QD should be performed under close medical supervision. Our study demonstrated that AUC and trough concentration level curves showed a strong correlation in patients who underwent allogeneic HSCT. The whole-blood trough should be maintained above 7.5ng/ml to provide an adequate level of AUC. If the trough level of Tac QD can be maintained above 7.5 ng/ml, Tac QD can be as effective as Tac iv and stable administration can be maintained for the patients with reduced stress because of the easier administration schedule. Our findings indicate that the use of Tac QD instead of Tac BID for GVHD prophylaxis is beneficial for patients undergoing allogeneic HSCT without moderate toxicities.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal