Abstract
Abstract 273
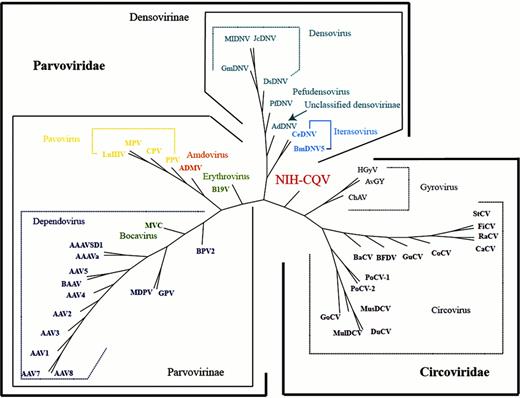
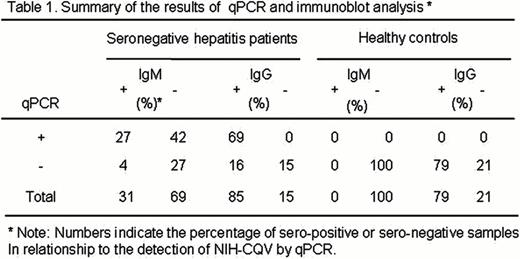
Seronegative hepatitis—non-hepatitis A, non-B, non-C, non-E—is poorly characterized but strongly associated with serious complications, especially aplastic anemia and fulminant hepatitis of childhood. Seronegative hepatitis is rare in the United States but more prevalent in Asia, constituting about 10–20% of acute cases. We applied next-generation sequencing to blood samples of patients from western China with seronegative hepatitis for virus discovery. A total of 92 plasma specimens were collected at Chongqing, China, between 1999 and 2007. Twenty-seven patients were diagnosed as having acute hepatitis by clinical and laboratory characteristics. Sixty-five patients had biopsy-proven chronic aggressive hepatitis, ten of which had cirrhosis. Serologic assays for hepatitis viruses A, B, C, E, HIV, Epstein-Barr virus and cytomegalovirus were all negative. Additional tests for antinuclear antibody, rheumatoid factor, anti-mitochondrial antibody also were normal. Ten plasma pools derived from 93 specimens of the patients were screened by Solexa deep sequencing. We discovered a 3780-bp contig present in all ten-pools that yielded tBLASTx E scores of 0.003 to 1.5 against parvoviruses. The sequence of the in silico assembled 3780-bp contig was confirmed by overlapping PCRs, indicating the contig that contained the nearly complete new virus genome indeed existed in the patient samples rather than being artificially generated by misassembly. The new virus is provisionally designated NIH-CQV. Further analysis revealed that the contig was composed of two major open reading frames (ORF). Protein Blast showed that ORF1 encoded a protein that contained a conserved P-loop NTPase domain, homologous to the replication-associated protein of bat circovirus (E score=4e-04). ORF2 was homologous to capsid protein of porcine parvovirus (E scores=7e-06). Phylogenetic analysis indicated that the NIH-CQV represents a new subfamily of parvovirus, located at the interface of Parvoviridae and Circoviridae (Figure 1). Prevalence of the NIH-CQV in hepatitis patients was investigated by qPCR. Sixty three out of 92 (69%) patient samples were positive, while all 45 healthy controls were negative. The average virus titer in the patients was 1.28 E4 copies/ul, and the highest one was 3.2 E4 copies/ul. Specific antibodies against NIH-CQV were sought by immunoblot using a recombinant capsid protein. No cross reactivity was detected between the capsid protein of NIH-CQV and other major human parvoviruses. Eighty five percent (78/92) of patients were positive for IgG, and 32% (29/92) of them were positive for IgM. In contrast, 78% (35/45) of healthy controls were positive for IgG and 16% (7/45) were positive for IgM. Viral particles were purified from IgM-positive patient plasma by ultracentrifugation through a 40% sucrose cushion and examined by electron microscopy: spherical, naked, parvovirus-like particles approximately 26–29 nm in diameter were visualized. There was no correlation between clinical diagnosis and the presence or absence of the viral DNA or specific antibodies. Although more work is needed to determine the etiologic role of NIH-CQV in human disease, our data indicate that a novel parvovirus-like virus is highly prevalent in a cohort of patients with seronegative hepatitis.
whole-proteome tree of the new parvovirus and members of the families Parvoviridae and Circoviridae.
whole-proteome tree of the new parvovirus and members of the families Parvoviridae and Circoviridae.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal