Abstract
Abstract 2432
Acute myeloid leukemia (AML) is a genetically and morphologically heterogeneous disease characterized by the accumulation of immature myeloid lineage cells in the bone marrow and blood. It results from genetic alterations that cause increased self-renewal of myeloid progenitors, accompanied by a block in their normal differentiation programs. Studies in mice and humans have shown that loss of expression of PU.1, a master transcription factor that is critical for lymphoid and myeloid lineage development, is a recurrent feature of AML1. Restoring the PU.1 differentiation program in AML is an attractive therapeutic strategy, but remains elusive due to a poor understanding of PU.1 target genes and tumor suppressive mechanisms. In a novel approach to understanding PU.1 function, we have used in vivo RNA interference to inducibly inhibit and restore PU.1 expression in normal hematopoietic cells and leukemias.
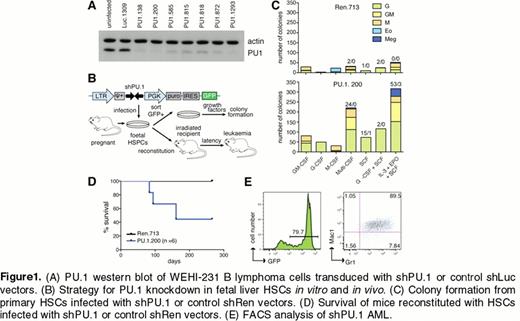
We identified several short hairpin RNAs that can effectively knockdown PU.1 (Fig 1A). We infected primary fetal liver cells with the most effective LMP-shPU.1 retroviruses and performed in vitro and in vivo assays to assess the effect of PU.1 knockdown (Fig 1B). We found that PU.1 knockdown drives 1) an increased frequency of blast colony-forming cells and self-generation of granulocytic progenitors in vitro (Fig 1C) and 2) a GFP+ myeloid leukemia after several months characterized by accumulation of cKit+Gr1+Mac1+ cells (Fig 1D, E). These findings verify that shRNA-mediated PU.1 knockdown can effectively disable its tumor suppressive functions.
To identify transcriptional targets of PU.1 in vivo, we utilized a recently generated reversible RNAi strategy that allows acute restoration of endogenous PU.1 expression upon Dox treatment in leukemias driven by PU.1 knockdown2. This TRMPV vector strategy allows tet-regulated co-expression of an shRNA and the fluorescent marker dsRed, with stable expression of GFP to mark infected cells. We transduced fetal liver cells derived from Vav-tTA transgenic mice with TRMPV-shPU.1 to drive reversible PU.1 knockdown across the hematopoietic system of reconstituted recipient mice. In contrast to the myeloid leukemia generated earlier using LMP-shPU.1, these mice developed pre-B cell (CD19+CD25+) leukemia with a latency of several months. To acutely restore endogenous PU.1 expression in leukemia, primary tumor cells were transplanted into several recipient mice to generate a cohort for analysis of Dox responses (Figure 2A). We found that dsRed intensity decreased incrementally upon Dox treatment of leukemic transplant recipient mice allowing FACS sorting of leukemia cells from triplicate untreated mice (dsRedhigh, minimal PU.1 expression) or after three days of Dox treatment (dsRedmid, partially restored PU.1 expression). We identified gene expression changes associated with PU.1 restoration using RNA sequencing (RNA-seq).
To further investigate PU.1 target genes in vivo, we have recently generated TRE-GFP-shPU.1 transgenic mice allowing inducible knockdown and restoration of PU.1 in adult mice. To test this strain we crossed it to CAGs-rtTA3 mice and treated bitransgenic mice with Dox. Western blot analysis of GFP+ Gr1+Mac1+ sorted myeloid cells showed effective PU.1 knockdown in vivo. We are currently using these mice to identify PU.1 regulated genes in normal myeloblasts in vivo.
These studies have identified several new candidate PU.1-regulated genes. Further experiments may shed light on whether there is a common novel tumor suppressive mechanism for PU.1 in myeloid and lymphoid leukemias driven by loss of PU.1.
No relevant conflicts of interest to declare.
References
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal