Abstract
Abstract 2343
Lifelong blood production is maintained by a very rare population of self-renewing hematopoietic stem cells (HSCs) in bone marrow (BM). Proliferation, differentiation and survival of HSC toward stepwise hematopoietic cell development needs to be tightly controlled by cell intrinsic and extrinsic factors, as excess or insufficient production of mature blood cells potentially leads to neoplasia or aplasia. HSCs and progenitors (HSPCs) are equipped with cell surface receptors for different cytokines or chemokines (Kaushansky, NEJM 2006), and thus can integrate external signals, leading finally to proliferation and subsequent increase of hematopoietic cells in demand. Some of these regulatory pathways are already exploited in clinical settings: CXCR4 antagonists for HSPC mobilization, human granulocyte colony-stimulating factor (hG-CSF) for HSC mobilization and myeloid regeneration, and thrombopoietin agonists (THPO) for improving thrombocytopenia. However, despite their clinical use, little is known about the influence of these molecules on HSC.
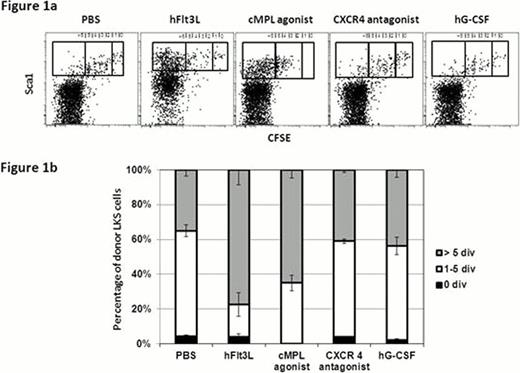
We established in vivo HSC divisional tracking with CFSE (5(6)-carboxyfluorescein diacetate N-succinimidyl ester). This allows to track single HSC division with high resolution, and subsequently to test biological activity of HSC-containing fractions (LKS) with different divisional history (Takizawa et al., JEM 2011). Using this system we evaluated the effects of systemic administration of human fms-related tyrosine kinase 3 ligand (hFlt3L), hG-CSF (Filgrastim), CXCR4 antagonist (AMD3100), and the THPO receptor (cMpl) agonist (Romiplostim) on HSC division. CFSE-labeled LKS were transferred into non-irradiated steady-state recipients. The non-dividing cell fraction was defined by the CFSE profile of CD4+CD62L+ T cells. One week after transplantation mice were injected with PBS or respective reagents daily or every other day for over one week. Three weeks after transfer, phenotypic BM analysis demonstrated that most of donor LKS had undergone several divisions while a small fraction of LKS remained undivided in PBS treated control mice (Figure 1a), containing long term self-renewing HSCs with at least 20–30% frequency (Takizawa et al., JEM 2011). Administration of hFlt3L increased the percentage of intermediate (1–5x divided) and fast cycling (>5x divided) LKS, which mainly contains CD150- Flt3+ multipotent progenitor cells (Figure 1b). Upon injections of cMpl agonist all donor LKS divided more than once, leaving no cells in quiescent fraction, with substantial expansion of CD150+ cells in the divided fraction. CXCR4 antagonist and hG-CSF administration had little effect on LKS proliferation. These data suggest that cMpl agonist drives dormant cells into proliferation, whereas hG-CSF has little effect on LKS division.
To determine whether cMpl agonist increases the turnover of functionally defined, bona fide HSCs, we performed secondary transplantation of 0–1, 2–4, and ≥5x divided LKS. Twenty fast- (≥5x divided cells at 3 weeks), slow-cycling (2–4x divided) or relatively dormant LKS Flt3- cMpl+ cells (0–1x divided) were sorted from mice treated with PBS or cMpl agonist, and transplanted into lethally irradiated mice. Early results demonstrate increased percentage of secondary recipient engrafted with 2–4x divided cells from primary animals treated with cMpl agonist compared to those cells from PBS treated control
Our results thus suggest that cMpl agonists have mitogenic activity not only for megakaryocyte progenitors but also for HSCs. How far this holds true in the human species needs to be determined. However, it should be taken in consideration given clinical data on evolution of pre-existing clonal myeloid diseases under cMpl agonist treatment (Dantoni, ASH abstract 2011), and also when treatment is applied long-term to patients with primary non-clonal hematopoietic diseases as immune thrombocytopenia or aplastic anemia.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal