Abstract
High-dose melphalan (HDM) for myeloma is the commonest conditioning regimen used in autologous transplantation with >10,000 transplants performed world-wide each year. Despite evidence of a dose-response effect, the standard dosing algorithm of 200mg/m2, with reduction to 140mg/m2 for renal impairment, has been based upon small comparative and observational studies, and not from pharmacokinetic analysis. We have examined the pharmacokinetics and clinical outcome of 115 patients receiving HDM and shown that exposure (area under the concentration versus time curve, AUC) above the median (12.85 mg/L.h) is associated with increased mucositis (HR 1.2, p < 0.005), but improved time to progression (TTP) (HR 0.35, p<0.02), and overall survival (OS), (HR 0.35, p = 0.006) at 2 years (91% versus 78%) [1]. We now compare the characteristics of patients with low and high AUCs to better identify those at risk of low drug exposure. We also sought to identify if outcomes differed in normal weight and obese (body mass index (BMI), ≥30 mg/m2) patients, where dose reductions based on lean body mass may have contributed to inferior survival.
For the 115 myeloma patients, (median age 58 (35 –73) years) enrolled in this study between 2004 and 2010 [1], the median follow up is now 3.7 years. The Mann Whitney test was the used to compare the following characteristics between patients with low (<12.85 mg/L.h) and high (≥12.85 mg/L.h) AUC and between obese and normal weight patients: weight; BMI; creatinine clearance (CrCl (ml/min/70 kg)); dose (mg/m2); % dose decrease (from 200 mg/m2); AUC and clearance. The influence of AUC on OS and TTP was re-analysed using Kaplan Meier survival analysis in all 115 patients and in the obese (n = 41). Significance was tested using the log rank test.
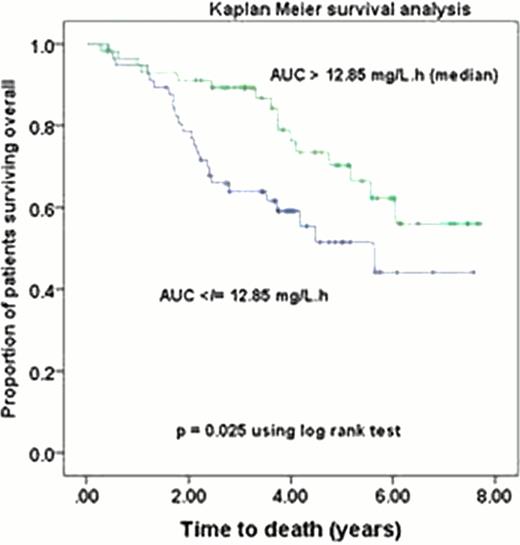
In the total population, patients with low and high melphalan AUC did not differ significantly in weight, BMI, mg/m2 melphalan dose or % dose decrease. However, patients with a low AUC had significantly better renal function: (CrCl, median (interquartile range) 97 (79–112) vs. 77 (58–90) ml/min/70 kg (p <0.001) and significantly higher melphalan clearance: 34 (31–40) vs. 24 (19–27) (p < 0.001). Similar significant results were observed in the obese population. Obese patients did not differ from normal weight patients in renal function, nor in melphalan AUC or clearance despite receiving a significantly (p=0.001) lower mg/m2 dose (190 (174 – 193) vs. 196 (187 – 199)). TTP and OS did not differ significantly between obese and normal-weight patients. Even with prolonged follow-up high AUC remained significantly associated with improved TTP (median 3.1 vs. 1.9yrs, p=0.041) and OS (median not reached vs. 5.6 yrs,) in the total population, (Fig 1). The 4yr OS was 76% vs. 59%, p=0.025). A high AUC was similarly associated with improved TTP (p=0.018) and OS (p=0.03) in the obese.
This pharmacokinetic study of HDM was conducted in the modern era characterised by both the impact of myeloma biotherapies and an increasing prevalence of obesity. Obese patients obtained comparable drug exposure and clinical outcomes as normal weight patients despite receiving a significantly lower mg/m2 dose of melphalan. Regardless of BMI, patients with low exposure to HDM had significantly better renal function, consistent with renal excretion being an important elimination pathway for melphalan. Given the significantly prolonged survival in patients with higher exposure to melphalan a dose increase may be appropriate in patients with excellent renal function. Clinical trials of pharmacokinetic based targeting to safely achieve this higher exposure, in lieu of a mg/m2 based dosing are now warranted.
No relevant conflicts of interest to declare.

This icon denotes a clinically relevant abstract
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal