Abstract
Abstract 178
CYT387 is a potent and selective small molecule inhibitor of JAK 1 and JAK 2 which is in clinical development for the treatment of myelofibrosis. Previously reported preliminary results from a phase I/II multicenter study demonstrated improvements in splenomegaly and constitutional symptoms as well as in RBC transfusion requirements. Enrollment has been completed and all subjects have now reached a minimum of 9 months on study. Updated safety and efficacy results are presented.
Subjects with high or intermediate-risk primary myelofibrosis (PMF) and post-polycythemia vera (post-PV) or post-essential thrombocythemia (ET) myelofibrosis were enrolled. Following an initial dose expansion phase, subjects were treated in a 9 month core study at an initial dose of 150 mg QD, 300 mg QD or 150 mg BID. Continued treatment with CYT387 was permitted in an extension phase for subjects who maintained at least stable disease. Responses were assessed by International Working Group (IWG) criteria with transfusion independence response defined as achieving a minimum 12 week transfusion-free period.
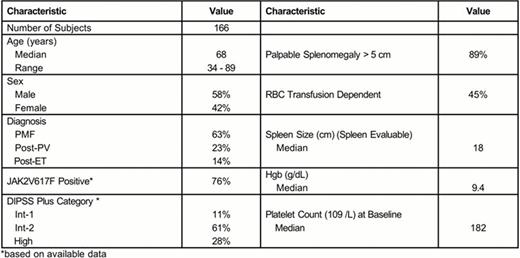
Enrollment of 166 subjects was completed at 6 study sites. Initial doses included 52 subjects at 150 mg QD, 60 subjects at 300 mg QD and 42 subjects at 150 mg BID. An additional 12 subjects were enrolled in other dose groups (100 mg QD, 200 mg QD, 400 mg QD) during the initial dose escalation phase. The median duration (range) of follow-up is 16.1 months (0.7 to 31.0 months).
Durable transfusion independence responses were observed in more than half of the RBC transfusion dependent subjects with a maximal transfusion-free period exceeding 2 years and ongoing. In addition, the percentage of all subjects requiring RBC transfusions substantially decreased over the treatment period. Treatment with CYT387 resulted in rapid and sustained reductions in splenomegaly with a maximal response duration approaching 2 years. The majority of subjects reporting constitutional symptoms at baseline experienced complete resolution or marked improvement by 6 months with measurable improvement within the first month of therapy. Higher transfusion independence and spleen response rates were seen in the 300 mg dose group compared to the 150 mg QD or 150 mg BID dose groups. For the first 60 consecutively enrolled subjects for whom the most mature data is available, the median follow-up period (range) is 21.5 months (2.9–31.0 months). The anemia and spleen response rates in these subjects, per IWG-MRT, were 59% and 48%, respectively; among 33 of these subjects who were RBC transfusion dependent by IWG-MRT criteria, 70% achieved a minimum 12-week period without transfusions with a maximal transfusion-free period of greater than 2 years and ongoing.
While 90% of subjects reported at least one treatment-related AE, the majority were reported as Grade 1. The most common treatment-related AEs were thrombocytopenia, peripheral neuropathy, dizziness, diarrhea, nausea, and headache. Treatment-related peripheral neuropathy was sensory, with almost all events reported as Grade 1. The most common Grade 3–4 treatment-related AEs included thrombocytopenia and hyperlipasemia. Only 5% of subjects reported treatment-related AEs resulting in study drug discontinuation. There were no treatment-related deaths.
CYT387 has proven safe and well tolerated even with prolonged administration for over 2.5 years. Treatment with CYT387 results in clinical improvement by effecting a rapid, meaningful and durable reduction of splenomegaly and the achievement of sustained RBC transfusion independence in a substantial number of subjects. CYT387 is also effective in improving constitutional symptoms. These results support the development of CYT387 at a dose of 300 mg QD for the treatment of myelofibrosis. Final analyses of safety and efficacy will be available at the time of the meeting.
Pardanani:Bristol-Myers Squibb: Clinical trial support, Clinical trial support Other; YM BioSciences: Clinical trial support, Clinical trial support Other; Sanofi-Aventis: Clinical trial support Other. Off Label Use: Data from the ongoing Phase-1/2 study of CYT387 use in myelofibrosis treatment will be described. Gotlib:YM Biosciences: Research Funding. Gupta:Celgene: Research Funding; Novartis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; YM Biosciences: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Sanofi-Aventis: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding; Incyte: Consultancy, Membership on an entity's Board of Directors or advisory committees, Research Funding. Roberts:YM BioSciences: clinical trial support Other. Wadleigh:Incyte: Membership on an entity's Board of Directors or advisory committees. Sirhan:Novartis: Consultancy, Honoraria. Bavisotto:YM BioSciences: Consultancy. Kawashima:YM BioSciences: Employment. Kowalski:YM BioSciences: Employment.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal