Abstract
Abstract 1722
Clofarabine is a nucleoside analogue that has shown activity in pts with myeloid disorders. The outcome of pts with MDS and CMML post clofarabine is unknown.
To evaluate the outcome of pts with MDS or CMML treated and failed clofarabine based chemotherapy as a first line or salvage regimen.
We reviewed data of 89 pts with MDS and 21 patients with CMML treated with a clofarabine based chemotherapy at MDACC between 6/2001 and 5/2012. Thirty-nine pts (36%) received clofarabine as first line therapy and 71 pts (64%) received clofarabine as salvage therapy. Ninety-six pts (87%) received single agent clofarabine and 14 pts (13%) received a clofarabine containing combination. Response assessment followed standard criteria. Overall survival (OS) was measured from the time of therapy till the time of death or last follow-up.
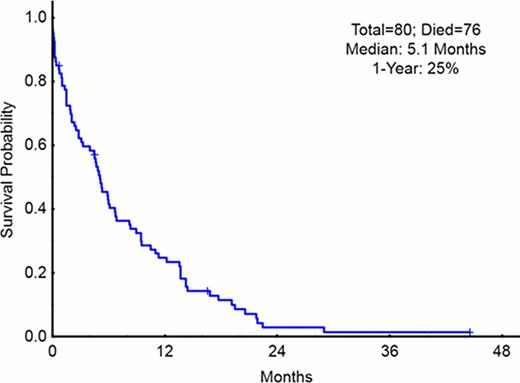
One hundred and ten pts with a median age of 68 years (range, 42–88) were assessed. Sixty-five percent of the pts were older than 65 years. At the time of MDS diagnosis, 68 (61%) had a high or intermediate-2 risk International Prognostic System Score (IPSS). Thirty-one pts (27%) had complex cytogenetics. Sixty-one pts (55%) had failed therapy with a hypomethylating agent (HMA) before initiation of a clofarabine containing salvage therapy. Of the pts who received frontline clofarabine treatment, 15 (23%) achieved complete remission (CR) and 2 (3%) CR with incomplete platelet recovery (CRp), for an overall response rate (ORR) of 26%. Of the pts who received salvage clofarabine treatment, 4 (9%) achieved CR and 5 (11%) CRp, for an ORR of 20%. The median duration of response to clofarabine was 6 months. ORR was 14% among the 61 patients who had received a prior HMA. At time of clofarabine failure, 20 (18%) had progressed to acute myelogenous leukemia (AML). Fifty-eight patients received salvage therapy after clofarabine failure. Among these, 13 patients received allogeneic stem cell transplant, 17 patients received a high dose Ara-C containing regimen and 25 patients received only investigational treatments. Only 8 (14%) responded (median duration of response not reached) (range, 0–40 months). Within a median follow-up of 3 months from clofarabine failure, 13 pts (14%) remained alive. The median OS post clofarabine failure was 5.1 months and the 1-year survival rate of 25% (figure 1).
Outcome of patients with MDS post clofarabine failure is poor, with a median survival of 5.1 months. These patients should be offered investigational strategies.
Kaplan Meier curve for patients with MDS after failure of clofarabine therapy.
Kaplan Meier curve for patients with MDS after failure of clofarabine therapy.
Off Label Use: Use of clofarabine is investigational in MDS.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal