Abstract
Abstract 140
Arsenic trioxide increases the rate of complete remission (CR) and prolongs long-term overall survival in patients with acute promyelocytic leukemia (APL). We demonstrated that an oral arsenic-containing Realgar-Indigo naturalis formula (RIF) could yield clear synergistic effects in an in vivo murine APL model as well as an in vitro APL cell lines. The efficacy and safety of this formula were confirmed by a CR rate of 96.7% and reasonable safety profile in a multicenter Phase II clinical trial in China. To find out whether oral RIF could achieve similar remission and survival with ATO, the Chinese APL Cooperative Group conducted a phase 3 randomized study (APL07) that compared oral RIF with ATO in both the induction and maintenance therapies.
In this phase 3 randomized trial, 242 newly diagnosed APL patients were randomly assigned to receive oral RIF (n=121, daily 60 mg per kg body weight) or ATO (n=121, daily 0.15 mg per kg body weight) as a part of induction therapy. All patients also received all-trans-retinoic acid (ATRA; daily 25 mg per square meter) as induction therapy, and were between the ages of 15 and 60 years. After achieving CR, all patients were given three courses of consolidation chemotherapy with anthracycline and cytosine arabinoside. The maintenance treatment included eight cycles of sequential use of ATRA and RIF or ATO for two years. The primary endpoint was disease-free survival (DFS). The secondary endpoints were the rate of CR and overall survival (OS) and safety.
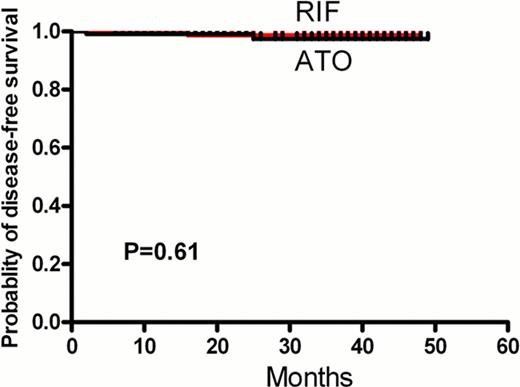
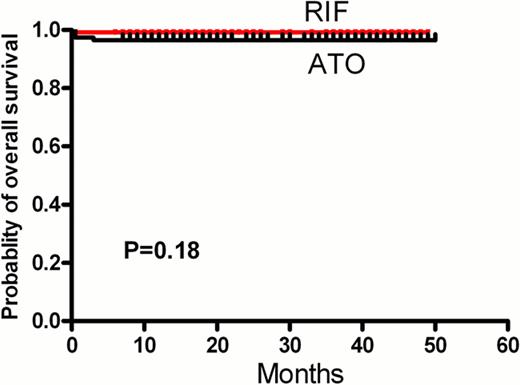
At a median follow-up of 28 months, no significant differences were noted between RIF group and ATO group with respect to CR rate (99.1% vs.97.2%, P=0.62), DFS (98.9% vs.97.64%, P=0.6) (Figure 1A) and OS at 3 years (99.1% vs.96.6%, P=0.18) (Figure 1B). The rates of adverse events were similar in the two groups. The rate of treatment-related grade 3/4 liver toxic effects was 9.6% in the RIF group and 12.0% in the ATO group(p=0.67). When on the arsenic-containing treatment, the plasma arsenic content was higher in the ATO group than in the RIF group (56.3 vs. 23.6 μg/L, p=0.0002). The median levels of arsenics concentration of plasm, urine, hair and nail samples after completing therapy 12 months were below the lower limit of the normal control.
Oral Realgar-Indigo naturalis formula yielded a comparable high remission and long-term survival with arsenic trioxide as front-line treatment of newly diagnosed APL.
The disease-free survival (A) and overall survival(B) of RIF group and ATO group.
A B
The disease-free survival (A) and overall survival(B) of RIF group and ATO group.
A B
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal