Abstract
Although patients with acute myeloid leukemia (AML) and the t(8;21) translocation generally have a favorable prognosis, relapse occurs in about 40% of cases and long-term (>5years) survival less than 50%. Patients with a KIT-mutation had an even higher relapse rate up to 70% and dismal survial. Once relapse, the outcome is extremely poor, even receiving allogeneic hematopoietic stem-cell transplantation (allo-HSCT).Therefore, rapidly identifying high-risk relapse patients and preemptively treating them with more aggressive therapy, such as HSCT, may decrease the chance of relapse and improve patient survival. We sought to improve outcome in patients with t(8;21) acute myeloid leukemia(AML) in first complete remission (CR) by applying risk-directed therapy that was based on measurements of minimal residual disease (MRD) by quantitative PCR during treatment.
From June 1,2005, to Dec 31, 2011, 137 patients with t(8;21) AML were enrolled at three centres. MRD was detected using quantitative PCR to detect the RUNX1/RUNX1T1 transcript. High-risk was defined by not achieving major molecular remission (MMR,> 3 log reduction of RUNX1/RUNX1T1 transcript from baseline) after second consolidation therapy or loss of MMR within 6 months since achieving MMR. Low-risk was defined by achieving MMR after second consolidation therapy and maintenance of MMR within 6 months thereafter. High-risk patients were recommended to receive allogeneic hematopoietic stem-cell transplantation (allo-HSCT) and low-risk patients to high-dose cytarabine-based consolidation chemotherapy. 116 patients who achieved CR and completed second consolidation were assigned to risk-directed therapy. Finally, sixty-nine patients actually received risk-directed therapy and 47 patients received a non risk-directed treatment for patients¡ bias.
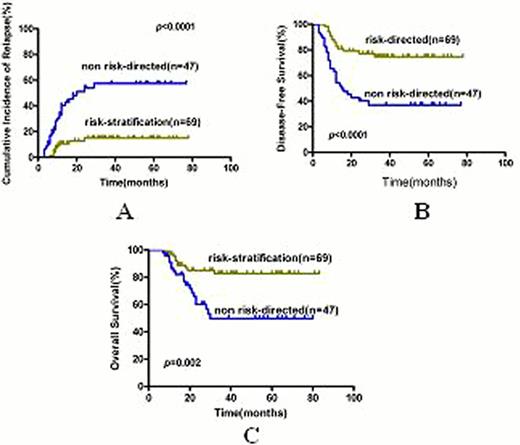
With a median follow-time of 36 months in patients alive, risk-directed therapy and non risk-directed therapy achieved 5 year cumulative incidence of relapse(CIR) of 15.0%±4.7% and 57.5%±8.0%(p<0.0001), disease-free survival(DFS) of 74.7%±5.8% and 37.1%±7.4%(p<0.0001) and overall survival (OS) of 82.7%±5.1% and 49.8%±8.5% (p=0.002) (Figure 1). Allo-HSCT benefited high-risk as well as KIT-mutated but impaired low-risk patients' DFS and OS (all p<0.05) (Figure 2). Multivariate analysis revealed that MRD status (high-risk vs. low-risk) and treatment (risk-directed vs. no risk-directed) were independent prognostic factor for relapse(hazard ratio 8.85, 95% CI 2.05–38.13, p=0.003; 0.26, 95% CI 0.12–0.61, p=0.002), DFS(hazard ratio 9.32, 95% CI 2.21–39.3; p=0.002; 0.36, 95% CI 0.17–0.75, p=0.007) and OS (hazard ratio10.53, 95% CI 1.41–78.83; p=0.022; 0.37, 95% CI 0.15–0.93, p=0.035).KIT-mutation was an independent prognostic factor for relapse(hazard ratio 2.12, 95% CI 1.01–4.48, p=0.049) but not for DFS and OS.
Risk-stratification treatment directed by MRD could improve the outcome of AML with t(8;21) in first complete remission. Allo-HSCT benefits high-risk as well as KIT-mutated but impairs low-risk patients¡ survival.
The outcomes of high-risk patients received allo-HSCT or chemotherapy(CT) as postremission treatment.
The outcomes of high-risk patients received allo-HSCT or chemotherapy(CT) as postremission treatment.
No relevant conflicts of interest to declare.

This icon denotes a clinically relevant abstract
Author notes
Asterisk with author names denotes non-ASH members.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal