Abstract
Abstract 1239
Hematopoiesis is a coordinated process in which hematopoietic stem cells (HSCs) undergo self-renewal and differentiation to produce multilineage blood cells. Maintenance of the HSC compartment has been shown to require regulation by a diverse profile of cytokines including members of the receptor tyrosine kinase (RTK) superfamily, with thrombopoietin (TPO) identified as a central regulator of HSC fate. HSC regulation is also mediated by serine-threonine kinases, such as transforming growth factor-beta (TGF-beta). Improved understanding of the mechanisms regulating HSC proliferation, self-renewal, and quiescence is essential for improving transplantation, ex vivo expansion, and other therapeutic applications of these cells.
Mammalian target of rapamycin (mTOR) is a central regulator of cellular metabolism, nutrient sensing and autophagy. mTOR has also emerged as a central regulator of HSC proliferation and self-renewal. Mouse models of mTOR hyperactivation demonstrate an imbalance of HSC proliferation and self-renewal leading to exhaustion of repopulating cells in a rapamycin-dependent manner, and pretreatment of human cord blood CD34+ cells with rapamycin has been shown to increase engraftment in immunodeficient mice. However, the mechanisms responsible for mTOR regulation of HSCs remain poorly understood.
Here we show that inhibition of mTOR by rapamycin significantly enhances signal transduction through both the TPO and TGF-beta pathways by inhibiting receptor endocytosis, resulting in increased cell surface levels of growth factor receptors and enhanced engraftment in a mouse model of prenatal HSC transplantation.
We employed primary and immortalized HSCs, as well as 293T cells stably overexpressing the thrombopoietin receptor, to study the effects of mTOR inhibition. Cells were treated with rapamycin for 72 hours prior to growth factor stimulation, and activation of signaling proteins were measured by immunoblotting and immunofluorescence studies. Surface receptor levels were determined by biotinylation and streptavidin immunoprecipitation. Clathrin- and caveolin-dependent endocytosis was inhibited by potassium depletion and nystatin treatment, respectively. Bone marrow transplantation studies were preformed in a well-characterized mouse model of in utero hematopoietic cell transplantation at 14 days gestational age, with engraftment quantified at 96 hours and 2 weeks postnatally.
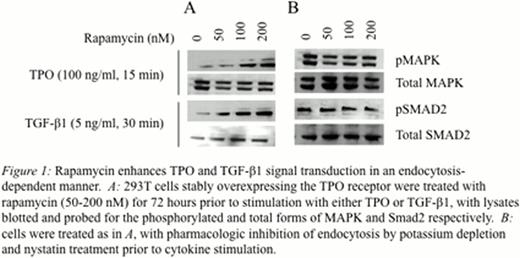
Rapamycin treatment resulted in enhanced signal transduction through both the TPO and TGF-beta pathways, with significantly increased levels of phosphorylation and nuclear translocation of MAPK and Smad proteins respectively (Figure 1A). Biotinylation studies revealed increased levels of cell surface receptors following mTOR inhibition. Pharmacologic disruption of endocytosis was found to ablate the effects of rapamycin treatment, with equivalent and robust activation of MAPK and Smad proteins in rapamycin-treated and untreated cells (Figure 1B). To test the hypothesis that enhanced cytokine signaling would result in an optimal profile of HSC function, in utero transplantation of rapamycin-treated primary bone marrow cells was performed. Rapamycin treatment resulted in enhanced HSC engraftment levels at 96 hours post-transplantation (4.2 +/− 0.6% vs 23.8 +/− 2.2%, p<0.005), and postnatal chimerism levels assessed at 2 weeks of age were similarly found to be significantly higher in the rapamycin-treatment group compared to controls (2.2 +/− 0.6% vs 29.8 +/− 10.7%, p<0.005).
These results support a role for mTOR in modulating HSC signal transduction by regulation of cytokine receptor endocytosis, resulting in enhanced levels of engraftment following transplantation. The observed effects of mTOR inhibition on multiple diverse cytokine profiles suggests a central mechanism of regulation of intracellular trafficking, which may be mediated at the level of formation of early endosomal and autophagosome complexes. Further studies will delineate receptor fate following internalization by these pathways. These studies will ultimately improve understanding of how the manipulation of multiple signaling circuits may be optimized to control self-renewal and quiescence compatible for in vitro expansion and transplantation of HSCs.
No relevant conflicts of interest to declare.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal