Abstract
Chemokines regulate the migration of hemopoietic cells and play an important role in the pathogenesis of many immune-mediated diseases. Intradermal recruitment of CD8+ T cells by CXCL10 is a central feature of the pathogenesis of cutaneous acute GVHD (aGVHD), but very little is known about the pathogenesis of chronic GVHD (cGVHD). Serum concentrations of the 3 CXCR3-binding chemokines, CXCL9, CXCL10, and CXCL11, were found to be markedly increased in patients with active cGVHD of the skin (n = 8). An 80% decrease in CD4+ cells expressing CXCR3 was seen in the blood of these patients (n = 5), whereas CD4+ cells were increased in tissue biopsies and were clustered around the central arterioles of the dermis. The well-documented increase in expression of CXCL10 in aGVHD therefore diversifies in cGVHD to include additional members of the CXCR3-binding family and leads to preferential recruitment of CD4+ T cells. These observations reveal a central role for chemokine-mediated recruitment of CXCR3+ T cells in cGVHD.
Introduction
GVHD is mediated by the development of a donor-derived alloreactive immune response and remains an important complication after allogeneic stem cell transplantation (SCT). Classic acute GVHD (aGVHD) typically presents with an acute onset of skin rash, diarrhea, and/or hepatic impairment, with a median onset of approximately 30 days after transplantation. In contrast, chronic GVHD (cGVHD) is a distinct clinical entity with a different profile of target tissues that commonly includes the skin, the oral and ocular mucosae, joints, and viscera.1 The importance of cGVHD is that it can result in an increased risk of nonrelapse mortality and overall survival and can lead to long-term patient disability and poor quality of life.2 Up to 50% of patients who undergo SCT develop symptoms of cGVHD.3 Treatments for cGVHD are limited and rely largely on long-term administration of immunosuppressant drugs, which can predispose patients to an increased risk of infection and disease relapse. New therapeutic approaches are therefore required but will depend on a more comprehensive understanding of disease pathogenesis.
The pathophysiology of aGVHD is relatively well established and is believed to result primarily from the induction of a CD8+ alloreactive immune response against host alloantigen. In contrast, much less is known regarding the pathophysiology of cGVHD. Animal models of cGVHD are relatively difficult to establish, but murine data suggest that CD4+ T cells have a primary role in disease pathogenesis.4-6 The role of regulatory T cells (Tregs) in the pathogenesis of cGVHD is currently under debate. Zorn et al illustrated a reduction in the frequency of peripheral Tregs in patients with mild cGVHD,7 and Matsuoka et al suggested that a relative deficiency of peripheral Tregs is associated with altered Treg homeostasis.8 However, literature regarding Tregs is still controversial, with a reduction in CD4+CD25highCD127low Tregs being associated with aGVHD but not cGVHD.9 Clarke et al reported an increase of peripheral Tregs in patients with cGVHD.10 More recently, Th17+ T cells have also been implicated in the pathogenesis of the disease.11,12
The role of chemokines in the development of GVHD is receiving increasing attention. The chemokine family is a large group of related proteins that play an important role in regulating the migration of hemopoietic cells. In particular, the recruitment and retention of T cells into specific tissues is thought to be mediated largely by the pattern of local chemokine expression and the profile of chemokine receptor proteins at the T-cell surface. Several previous studies have illustrated a role for chemokines and their receptors in T-cell infiltration of the tissues in aGVHD.13 For example, both CCL27 (CTACK)–CCR10 and CXCL10-CXCR3 interactions have been associated with recruitment of T cells in aGVHD.14,15 There has been no study of systemic chemokine levels in the setting of cGVHD, although local concentrations of CXCL9 are increased in cGVHD of the oral mucosa and CXCR3 was shown to be expressed on the infiltrating T cells.16 Increased levels of these Th1-associated chemokines were also seen in 10 patients with cGVHD of the conjunctiva.17
In the present study, we examined the systemic and local importance of chemokine-mediated migration in a large cohort of patients with cGVHD. We observed that increased levels of the Th1-associated chemokines CXCL9, CXCL10, and CXCL11 led to recruitment of CXCR3+ T cells from the peripheral blood into affected tissues. A striking predominance of CD4+ T cells was observed within intradermal T-cell populations, indicating a novel role for the Th-1–mediated HLA class II–restricted processes in disease progression.
Methods
Luminex cytokine analysis
For Luminex cytokine analysis, a total of 46 patients who had undergone SCT were selected. Of these, 24 received a graft from an HLA-matched sibling, 21 from an unrelated donor, and 1 from a cousin. Patients underwent either a myeloablative conditioning regimen or a nonmyeloablative regimen (Table 1). Patients were recruited after written informed consent in accordance with the Declaration of Helsinki as approved by the South Birmingham Research Ethics Committee (Q5/Q2707/175). GVHD patients were divided into 2 groups based on whether they exhibited selective cGVHD of the skin.
Patient characteristics for Luminex analysis including control SCT patients
| Group . | Patient ID . | Disease . | Transplantation date . | Time after transplantation . | Conditioning . | Transplantation type . | Alem . | GVHD prophylaxis . | Stem cell source . | NIH classification (retrospective) . | Tissue affected . | Treatment . | Previous aGVHD . | Grade . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Non-skin | MW342 | AML | 23-May-06 | 313 wk | Cy TBI 14.4 Gy | SIB | None | CSA + MTX | PBSCs | Classic | Joints | CSA + increase prednisolone | None | N/A |
| DC331 | AML | 10-Feb-09 | 20 wk | Flu/Mel/Alem, no TBI | MUD | In vivo | CSA only | PBSCs | Classic | Gut | Increase prednisolone | Skin | I | |
| FP282 | AML | 04-Mar-08 | 20 wk | Cy TBI 14.4 Gy | MUD | In vivo | CSA + MTX | PBSCs | Classic | Oral | Oral prednisolone | Lower gut | Not known | |
| PM341 | CML | 26-Jan-07 | 121 wk | Cy TBI 14.4 Gy | MUD | In vivo | CSA + MTX | PBSCs | Classic | Oral | CSA + increase prednisolone | None | N/A | |
| YA015 | CLL | 15-Feb-08 | 82 wk | Reduced intensity T replete | MUD | None | CSA only | PBSCs | Classic | Liver | CSA | None | N/A | |
| CS213 | MM | 28-Mar-06 | 42 wk | Mel TBI 12.0 Gy | SIB | None | CSA + MTX | PBSCs | Classic | Oral | Topical/systemic prednisolone | Skin | III | |
| AE303 | AML | 22-Jul-08 | 43 wk | Cy TBI 14.4 Gy | SIB | None | CSA + MTX | PBSCs | Classic | Lung | Prednisolone | No | N/A | |
| MP328 | CML | 27-Jan-09 | 25 wk | Bu/Cy | SIB | None | CSA + MTX | PBSCs | Late aGVHD | Liver | Increased CSA and prednisolone | Skin, lower gut | II | |
| CW254 | NHL | 12-Jun-07 | 22 wk | Bean/Alem | SIB | In vivo | CSA + MTX | PBSCs | Late aGVHD | Oral and Ocular | Nil | Skin | II | |
| ML375 | AML | 19-Nov-09 | 21 wk | Flu/Mel/Alem, no TBI | MUD | In vivo | CSA only | PBSCs | Late aGVHD | Gut | Methyl prednisolone + prednisolone | Lower gut | IV | |
| BC350 | AML | 07-Jul-09 | 16 wk | Cy TBI 14.4 Gy | SIB | None | CSA + MTX | PBSCs | Late aGVHD | Liver | Prednisolone | Liver | II | |
| SR368 | ALL | 24-Mar-09 | 29 wk | Cy TBI 14.4 Gy | SIB | None | CSA + MTX | PBSCs | Classic | Oral | Oral prednisolone | No | N/A | |
| AH304 | MF | 05-Aug-08 | 88 wk | Bu/Cy | SIB | None | CSA + MTX | PBSCs | Classic | Lung | CSA + increased Prednisolone | No | N/A | |
| LD309 | MDS | 02-Sep-08 | 20 wk | Flu/Mel/Alem, no TBI | MUD | In vivo | CSA only | PBSCs | Late aGVHD | Gut | CSA only | Gut, skin | Unknown | |
| PH260 | AML | 18-Jul-07 | 28 wk | Flu/Mel/Alem, no TBI | MUD | In vivo | CSA only | PBSCs | Classic | Oral | Oral prednisolone | Skin | I | |
| LJ257 | NHL | 03-Jul-07 | 27 wk | Cy TBI 14.4 Gy | SIB | None | CSA + MTX | PBSCs | Classic | Oral, ocular, joints | Oral prednisolone, eye drops | Skin | I | |
| RG338 | AML | 01-Apr-09 | 21 wk | Flu/Mel/Alem, no TBI | MUD | In vivo | CSA only | PBSCs | Classic | Oral | CSA only | Skin | I | |
| AC231 | AML | 25-Oct-06 | 19 wk | Flu/Mel/Alem, no TBI | SIB | In vivo | CSA only | PBSCs | Classic | Oral | Oral prednisolone | Skin | I | |
| SC352 | MDS | 28-Jul-09 | 38 wk | Cy TBI 14.4 Gy | Cousin | None | CSA + MTX | PBSCs | Classic | Oral | CSA only | Gut | Not known | |
| JS319 | ALL | 18-Nov-08 | 16 wk | Cy TBI 14.4 Gy | SIB | None | CSA + MTX | PBSCs | Classic | Oral | Prednisolone mouthwash | Skin | I | |
| Skin | CM308 | AML | 28-Aug-08 | 50 wk | Flu/Mel/Alem, no TBI | MUD | In vivo | CSA only | PBSCs | Late aGVHD | Skin | CSA only | Gut | IV |
| SK322 | AML | 10-Dec-08 | 20 wk | Flu/Mel/Alem, no TBI | MUD | In vivo | CSA only | PBSCs | Late aGVHD | Skin | Betnovate cream | Skin, Gut | II | |
| TR382 | MF | 28-Apr-09 | 33 wk | Flu/Mel/Alem, no TBI | MUD | None | CSA only | PBSCs | Late aGVHD | Skin | Nil | Skin | II | |
| OT356 | AML | 18-Aug-09 | 25 wk | Flu/Mel/Alem, no TBI | MUD | In vivo | CSA only | PBSCs | Late aGVHD | skin | Topical prednisolone | Skin | I | |
| CL380 | AML | 06-May-09 | 31 wk | Flu/Mel/Alem, no TBI | MUD | In vivo | CSA only | PBSCs | Late aGVHD | Skin | Prednisolone and topical steroids | None | N/A | |
| JG226 | AML | 27-Sep-06 | 21 wk | Flu/Mel/Alem, no TBI | SIB | In vivo | CSA only | PBSCs | Classic | Skin | Topical prednisolone | Yes | Not known | |
| IS383 | AML | 12-Jan-10 | 20 wk | Flu/Mel/Alem, no TBI | MUD | In vivo | CSA only | PBSCs | Classic | Skin | Prednisolone | None | N/A | |
| RS373 | AML-secondary | 03-Nov-09 | 63 wk | Flu/Mel/Alem, no TBI | SIB | In vivo | CSA only | PBSCs | Late aGVHD | Skin | Increase CSA, topical prednisolone | Skin | I | |
| Control | DC224 | HL | 19-Sep-06 | 24 wk | Beam/Alem, no TBI | SIB | In vivo | CSA + MTX | PBSCs | N/A | No | N/A | None | N/A |
| SG203 | AML | 10-Jan-06 | 32 wk | Cy/TBI 14.4 Gy | SIB | None | CSA + MTX | PBSCs | N/A | No | N/A | None | N/A | |
| JT252 | AML | 06-Jun-07 | 72 wk | Flu/Mel/Alem, no TBI | SIB | In vivo | CSA only | PBSCs | N/A | No | N/A | None | N/A | |
| MP306 | NHL | 19-Aug-08 | 37 wk | Beam/Alem, 12.0 Gy | SIB | In vivo | Cy + MTX | PBSCs | N/A | No | N/A | None | N/A | |
| NG315 | AML | 28-Oct-08 | 25 wk | Flu/Mel/Alem, no TBI | MUD | In vivo | Cy only | PBSCs | N/A | No | N/A | None | N/A | |
| HM281 | AML | 04-Mar-08 | 36 wk | Cy/TBI, 14.4 Gy | SIB | None | CSA + MTX | PBSCs | N/A | No | N/A | Gut, Liver | N/A | |
| EH325 | AML | 13-Jan-09 | 17 wk | Cy/TBI, 14.4 Gy | SIB | None | CSA + MTX | PBSCs | N/A | No | N/A | None | N/A | |
| SS312 | AML | 14-Oct-08 | 25 wk | Cy/TBI, 14.4 Gy | SIB | None | CSA + MTX | PBSCs | N/A | No | N/A | None | N/A | |
| MM285 | HL | 11-Mar-08 | 26 wk | Beam/Alem, no TBI | SIB | In vivo | CSA + MTX | PBSCs | N/A | No | N/A | None | N/A | |
| LT265 | ALL | 04-Sep-07 | 51 wk | Cy/TBI/cranial boost, | SIB | None | CSA + MTX | PBSCs | N/A | No | N/A | None | N/A | |
| RN253 | ALL | 12-Jun-07 | 97 wk | Cy/TBI, 14.4 Gy | SIB | None | CSA + MTX | PBSCs | N/A | No | N/A | None | N/A | |
| RD335 | TC-NHL | 17-Mar-09 | 25 wk | Flu/Mel/Alem, no TBI | MUD | In vivo | CSA only | PBSCs | N/A | No | N/A | None | N/A | |
| RT330 | MCL | 10-Feb-09 | 16 wk | Flu/Mel/Alem, no TBI | MUD | In vivo | CSA only | PBSCs | N/A | No | N/A | None | N/A | |
| JJ258 | AML | 18-Jul-07 | 95 wk | Flu/Mel/Alem, no TBI | MUD | In vivo | CSA only | PBSCs | N/A | No | N/A | None | N/A | |
| JH370 | CLL | 20-Oct-09 | 22 wk | Flu/Mel/Alem, no TBI | MUD | In vivo | CSA only | PBSCs | N/A | No | N/A | None | N/A | |
| PP361 | NHL | 16-Oct-09 | 16 wk | Flu/Mel/Alem, no TBI | MUD | In vivo | CSA only | PBSCs | N/A | No | N/A | None | N/A | |
| SG220 | APML | 11-Jul-06 | 27 wk | Cy /TBI, 14.4 Gy | SIB | None | CSA only | PBSCs | N/A | No | N/A | Skin | N/A | |
| SS381 | ALL | 16-Dec-09 | 26 wk | Flu/Mel/Alem, no TBI | MUD | In vivo | CSA only | PBSCs | N/A | No | N/A | None | N/A |
| Group . | Patient ID . | Disease . | Transplantation date . | Time after transplantation . | Conditioning . | Transplantation type . | Alem . | GVHD prophylaxis . | Stem cell source . | NIH classification (retrospective) . | Tissue affected . | Treatment . | Previous aGVHD . | Grade . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Non-skin | MW342 | AML | 23-May-06 | 313 wk | Cy TBI 14.4 Gy | SIB | None | CSA + MTX | PBSCs | Classic | Joints | CSA + increase prednisolone | None | N/A |
| DC331 | AML | 10-Feb-09 | 20 wk | Flu/Mel/Alem, no TBI | MUD | In vivo | CSA only | PBSCs | Classic | Gut | Increase prednisolone | Skin | I | |
| FP282 | AML | 04-Mar-08 | 20 wk | Cy TBI 14.4 Gy | MUD | In vivo | CSA + MTX | PBSCs | Classic | Oral | Oral prednisolone | Lower gut | Not known | |
| PM341 | CML | 26-Jan-07 | 121 wk | Cy TBI 14.4 Gy | MUD | In vivo | CSA + MTX | PBSCs | Classic | Oral | CSA + increase prednisolone | None | N/A | |
| YA015 | CLL | 15-Feb-08 | 82 wk | Reduced intensity T replete | MUD | None | CSA only | PBSCs | Classic | Liver | CSA | None | N/A | |
| CS213 | MM | 28-Mar-06 | 42 wk | Mel TBI 12.0 Gy | SIB | None | CSA + MTX | PBSCs | Classic | Oral | Topical/systemic prednisolone | Skin | III | |
| AE303 | AML | 22-Jul-08 | 43 wk | Cy TBI 14.4 Gy | SIB | None | CSA + MTX | PBSCs | Classic | Lung | Prednisolone | No | N/A | |
| MP328 | CML | 27-Jan-09 | 25 wk | Bu/Cy | SIB | None | CSA + MTX | PBSCs | Late aGVHD | Liver | Increased CSA and prednisolone | Skin, lower gut | II | |
| CW254 | NHL | 12-Jun-07 | 22 wk | Bean/Alem | SIB | In vivo | CSA + MTX | PBSCs | Late aGVHD | Oral and Ocular | Nil | Skin | II | |
| ML375 | AML | 19-Nov-09 | 21 wk | Flu/Mel/Alem, no TBI | MUD | In vivo | CSA only | PBSCs | Late aGVHD | Gut | Methyl prednisolone + prednisolone | Lower gut | IV | |
| BC350 | AML | 07-Jul-09 | 16 wk | Cy TBI 14.4 Gy | SIB | None | CSA + MTX | PBSCs | Late aGVHD | Liver | Prednisolone | Liver | II | |
| SR368 | ALL | 24-Mar-09 | 29 wk | Cy TBI 14.4 Gy | SIB | None | CSA + MTX | PBSCs | Classic | Oral | Oral prednisolone | No | N/A | |
| AH304 | MF | 05-Aug-08 | 88 wk | Bu/Cy | SIB | None | CSA + MTX | PBSCs | Classic | Lung | CSA + increased Prednisolone | No | N/A | |
| LD309 | MDS | 02-Sep-08 | 20 wk | Flu/Mel/Alem, no TBI | MUD | In vivo | CSA only | PBSCs | Late aGVHD | Gut | CSA only | Gut, skin | Unknown | |
| PH260 | AML | 18-Jul-07 | 28 wk | Flu/Mel/Alem, no TBI | MUD | In vivo | CSA only | PBSCs | Classic | Oral | Oral prednisolone | Skin | I | |
| LJ257 | NHL | 03-Jul-07 | 27 wk | Cy TBI 14.4 Gy | SIB | None | CSA + MTX | PBSCs | Classic | Oral, ocular, joints | Oral prednisolone, eye drops | Skin | I | |
| RG338 | AML | 01-Apr-09 | 21 wk | Flu/Mel/Alem, no TBI | MUD | In vivo | CSA only | PBSCs | Classic | Oral | CSA only | Skin | I | |
| AC231 | AML | 25-Oct-06 | 19 wk | Flu/Mel/Alem, no TBI | SIB | In vivo | CSA only | PBSCs | Classic | Oral | Oral prednisolone | Skin | I | |
| SC352 | MDS | 28-Jul-09 | 38 wk | Cy TBI 14.4 Gy | Cousin | None | CSA + MTX | PBSCs | Classic | Oral | CSA only | Gut | Not known | |
| JS319 | ALL | 18-Nov-08 | 16 wk | Cy TBI 14.4 Gy | SIB | None | CSA + MTX | PBSCs | Classic | Oral | Prednisolone mouthwash | Skin | I | |
| Skin | CM308 | AML | 28-Aug-08 | 50 wk | Flu/Mel/Alem, no TBI | MUD | In vivo | CSA only | PBSCs | Late aGVHD | Skin | CSA only | Gut | IV |
| SK322 | AML | 10-Dec-08 | 20 wk | Flu/Mel/Alem, no TBI | MUD | In vivo | CSA only | PBSCs | Late aGVHD | Skin | Betnovate cream | Skin, Gut | II | |
| TR382 | MF | 28-Apr-09 | 33 wk | Flu/Mel/Alem, no TBI | MUD | None | CSA only | PBSCs | Late aGVHD | Skin | Nil | Skin | II | |
| OT356 | AML | 18-Aug-09 | 25 wk | Flu/Mel/Alem, no TBI | MUD | In vivo | CSA only | PBSCs | Late aGVHD | skin | Topical prednisolone | Skin | I | |
| CL380 | AML | 06-May-09 | 31 wk | Flu/Mel/Alem, no TBI | MUD | In vivo | CSA only | PBSCs | Late aGVHD | Skin | Prednisolone and topical steroids | None | N/A | |
| JG226 | AML | 27-Sep-06 | 21 wk | Flu/Mel/Alem, no TBI | SIB | In vivo | CSA only | PBSCs | Classic | Skin | Topical prednisolone | Yes | Not known | |
| IS383 | AML | 12-Jan-10 | 20 wk | Flu/Mel/Alem, no TBI | MUD | In vivo | CSA only | PBSCs | Classic | Skin | Prednisolone | None | N/A | |
| RS373 | AML-secondary | 03-Nov-09 | 63 wk | Flu/Mel/Alem, no TBI | SIB | In vivo | CSA only | PBSCs | Late aGVHD | Skin | Increase CSA, topical prednisolone | Skin | I | |
| Control | DC224 | HL | 19-Sep-06 | 24 wk | Beam/Alem, no TBI | SIB | In vivo | CSA + MTX | PBSCs | N/A | No | N/A | None | N/A |
| SG203 | AML | 10-Jan-06 | 32 wk | Cy/TBI 14.4 Gy | SIB | None | CSA + MTX | PBSCs | N/A | No | N/A | None | N/A | |
| JT252 | AML | 06-Jun-07 | 72 wk | Flu/Mel/Alem, no TBI | SIB | In vivo | CSA only | PBSCs | N/A | No | N/A | None | N/A | |
| MP306 | NHL | 19-Aug-08 | 37 wk | Beam/Alem, 12.0 Gy | SIB | In vivo | Cy + MTX | PBSCs | N/A | No | N/A | None | N/A | |
| NG315 | AML | 28-Oct-08 | 25 wk | Flu/Mel/Alem, no TBI | MUD | In vivo | Cy only | PBSCs | N/A | No | N/A | None | N/A | |
| HM281 | AML | 04-Mar-08 | 36 wk | Cy/TBI, 14.4 Gy | SIB | None | CSA + MTX | PBSCs | N/A | No | N/A | Gut, Liver | N/A | |
| EH325 | AML | 13-Jan-09 | 17 wk | Cy/TBI, 14.4 Gy | SIB | None | CSA + MTX | PBSCs | N/A | No | N/A | None | N/A | |
| SS312 | AML | 14-Oct-08 | 25 wk | Cy/TBI, 14.4 Gy | SIB | None | CSA + MTX | PBSCs | N/A | No | N/A | None | N/A | |
| MM285 | HL | 11-Mar-08 | 26 wk | Beam/Alem, no TBI | SIB | In vivo | CSA + MTX | PBSCs | N/A | No | N/A | None | N/A | |
| LT265 | ALL | 04-Sep-07 | 51 wk | Cy/TBI/cranial boost, | SIB | None | CSA + MTX | PBSCs | N/A | No | N/A | None | N/A | |
| RN253 | ALL | 12-Jun-07 | 97 wk | Cy/TBI, 14.4 Gy | SIB | None | CSA + MTX | PBSCs | N/A | No | N/A | None | N/A | |
| RD335 | TC-NHL | 17-Mar-09 | 25 wk | Flu/Mel/Alem, no TBI | MUD | In vivo | CSA only | PBSCs | N/A | No | N/A | None | N/A | |
| RT330 | MCL | 10-Feb-09 | 16 wk | Flu/Mel/Alem, no TBI | MUD | In vivo | CSA only | PBSCs | N/A | No | N/A | None | N/A | |
| JJ258 | AML | 18-Jul-07 | 95 wk | Flu/Mel/Alem, no TBI | MUD | In vivo | CSA only | PBSCs | N/A | No | N/A | None | N/A | |
| JH370 | CLL | 20-Oct-09 | 22 wk | Flu/Mel/Alem, no TBI | MUD | In vivo | CSA only | PBSCs | N/A | No | N/A | None | N/A | |
| PP361 | NHL | 16-Oct-09 | 16 wk | Flu/Mel/Alem, no TBI | MUD | In vivo | CSA only | PBSCs | N/A | No | N/A | None | N/A | |
| SG220 | APML | 11-Jul-06 | 27 wk | Cy /TBI, 14.4 Gy | SIB | None | CSA only | PBSCs | N/A | No | N/A | Skin | N/A | |
| SS381 | ALL | 16-Dec-09 | 26 wk | Flu/Mel/Alem, no TBI | MUD | In vivo | CSA only | PBSCs | N/A | No | N/A | None | N/A |
Shown are clinical features of control SCT patients and patients who went on to develop cGVHD after SCT. The average time of sample analysis after SCT was 37 weeks for controls, 33 weeks for skin only, 50 weeks for non-skin, and 42 weeks for all GVHD.
AML indicates acute myeloid leukemia; CML, chronic myeloid leukemia; CLL, chronic lymphocytic leukemia; MM, multiple myeloma; NHL, non-Hodgkin lymphoma; ALL, acute lymphocytic leukemia; MDS, myelodysplastic syndrome; MF, myelofibrosis; HL, Hodgkin lymphoma; TC-NHL, T-cell NHL; MCL, mantle cell lymphoma; APML, acute promyelocytic myelogenous leukemia; TBI, total body irradiation; Flu, fludarabine; Mel, melphalan; Alem, alemtuzumab; Cy, cyclophosphamide; SIB, sibling donor; MUD, matched unrelated donor; MTX, methotrexate; and PBSCs, peripheral blood stem cells.
Flow cytometry
For flow cytometric analysis of the peripheral blood, a panel of patients undergoing a nonmyeloablative, matched unrelated donor transplantation was selected. Patients with clinical symptoms of skin GVHD only were compared with patients who had no clinical symptoms of GVHD at the time of sample collection. Patients and controls were matched for time after transplantation.
All samples were selected retrospectively at the closest time point to onset of symptoms of GVHD and before changes in their GVHD treatment. For analysis of fresh 4-mm skin-punch biopsies, patients were assessed at the time of disease presentation. The classification of GVHD in this study was initially based on the Seattle criteria,18 which was in common use worldwide when our study was initiated. During the course of this study, the National Institutes of Health (NIH) consensus criteria were published, which classified cGVHD as being either classic chronic or overlap syndrome.19 A further category of persistent, recurrent or late aGVHD (to be referred to as “delayed aGVHD” in this manuscript) was also introduced to incorporate patients who present with typical features of aGVHD beyond 100 days after transplantation (often in the context of withdrawal of immune suppression). We have reclassified our patient cohort retrospectively according to the NIH criteria.
All patients diagnosed with grade I delayed aGVHD receive topical corticosteroids and those with delayed aGVHD of grades II-IV or with moderate/severe classic disease received systemic steroids in combination with continuation or addition of therapeutic levels of cyclosporin A (CSA). Individual treatments received by each patient are indicated in Table 1.
PBMCs and serum
Peripheral blood was taken into sodium heparin tubes or into heparin-free tubes for serum isolation. Peripheral blood was taken every 2 weeks after SCT until week 12, then at monthly intervals for up to 1 year and at 3 monthly intervals for up to 2 years. Additional samples were taken when patients presented with disease. Samples were then retrospectively selected from the time of cGVHD onset. PBMCs were isolated by density gradient centrifugation over Lymphoprep, frozen, and stored in liquid nitrogen. Serum was isolated and stored at −80°C until use.
Serum chemokine measurements
CXCL9, CXCL10, and CXCL11 were analyzed in serum using a multiplex fluorescent bead-based immunoassay (Affymetrix) and run on a Luminex 100 machine. Samples were analyzed using StarStation Luminex Version 2.3 software.
Skin biopsy analysis
Skin-punch biopsies (4 mm) were obtained from patients with aGVHD or cGVHD from the affected skin region and compared with punch biopsies taken from healthy control laboratory donors (HDs). All skin biopsies were taken by a qualified clinician or research nurse using an institutional review board–approved protocol (South Birmingham Research Ethics Committee). Biopsies were digested with collagenase D (Roche) for 1 hour at 37°C to obtain mononuclear cell populations. Cells were then incubated on plastic for 30 minutes in RPMI medium to remove debris, and the nonadherent cells were collected for flow cytometric analysis.
Flow cytometry
PBMC and skin biopsy T-cell populations were analyzed using multicolor flow cytometry. Up to 9-color flow cytometry was performed using CD3-APC-Cy7 (clone UCHT1; Cambridge Biosciences), CD4-ECD (clone T4; Beckman Coulter), CD8-AmCyan (clone SK1; BD Biosciences), CD45RA-AF700 (clone A100; Cambridge Biosciences), CCR7-FITC (clone 150503; R&D Systems), CCR4-PE-Cy7 (clone IG1; BD Biosciences), CCR6-PerCpCy5.5 (clone 11A9; BD Biosciences), CCR10-APC (clone 314305; BD Biosciences), and CXCR3-PE (clone 49801; R&D Systems). In brief, mononuclear cells were surface stained with Abs on ice for 20 minutes, protected from light, and compared with unstained controls. For Treg analysis, cells were surface stained with CD3-APC-Cy7 (clone UCHT1; Cambridge Biosciences), CD4-FITC (clone HIT3a; Beckman Coulter), and CXCR3-PerCpCy5.5 (clone 16C; BD Biosciences), permeabilized, and stained for intracellular FoxP3-PE expression (clone PCH101; BD Biosciences). Data were acquired on an LSR II flow cytometer (BD Biosciences) and analyzed using FACSDiva Version 6.1.3 software. For analysis of PBMCs, the lymphocyte population was selected, followed by gating on CD3+ T cells. CD3+ T cells were then analyzed for CD4 and CD8 expression or for CCR7 and CD45RA expression. For chemokine receptor analysis, receptors were expressed as a proportion of either CD3+ T cells for skin analysis or of CD4+/CD8+ T cells for peripheral blood analysis. For analysis of Tregs, lymphocytes were selected, and then CD4+CD3+ cells gated, followed by FoxP3 expression. CXCR3 was then expressed as a proportion of FoxP3+CD4+CD3+ cells.
Immunohistochemistry
Formalin-fixed, paraffin-embedded tissue biopsy sections from patients taken at the time of aGVHD or cGVHD were stained with murine IgG isotype control or with CD4 (clone 4B12; Vector Laboratories) or CXCR3 (clone IGC; R&D Systems) mAbs. Staining was compared with skin biopsies retrieved from HD controls who had undergone breast reduction surgery. In brief, sections were de-waxed and antigen retrieved by heating slides to 60°C, followed by incubation in W-Cap pH8 TECH buffer (Surgipath) for 30 minutes at 98°C and cooling for 20 minutes. Sections were washed and blocked for 10 minutes with 1% hydrogen peroxide in methanol, washed in TBS, and incubated with normal horse serum (ImmPress kit; Vector Laboratories) for 20 minutes. Sections were then incubated with the Abs of interest for 1 hour, washed with TBS-Tween 0.01%, and then incubated with secondary anti–mouse/rabbit peroxidase (ImmPress; Vector Laboratories). Staining was developed with DAB chromogen (ImmPACT; Vector Laboratories) and sections counterstained with hematoxylin.
Statistical analysis
All statistical analysis was performed using SPSS Version 19 software (IBM) or Prism Version 5.03 software (GraphPad). Comparisons were made for each experiment, as described in the figure legends. The Fisher exact test was used to assess the influence of transplantation characteristics on cGVHD incidence in our patient cohort. P < .05 was considered statistically significant.
Results
CXCL9, CXCL10, and CXCL11 levels are elevated in the serum of SCT patients who develop cGVHD
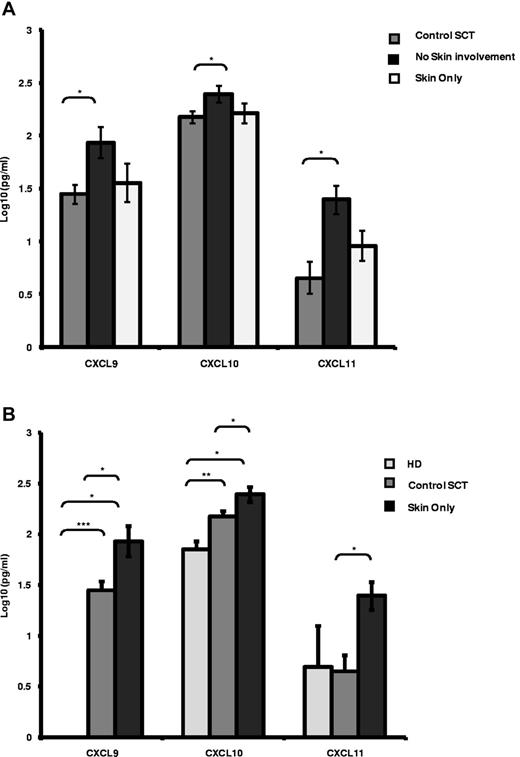
The levels of the 3 CXCR3-binding chemokines, CXCL9, CXCL10, and CXCL11, were measured in the serum of 28 patients at the time of cGVHD and compared with matched samples taken from allotransplantation patients without cGVHD (n = 18). Patients with cGVHD were subdivided into those with skin involvement only (n = 8) and those with organ involvement by cGVHD other than the skin (n = 20). In patients with skin-only cGVHD, the serum levels of CXCL9, CXCL10, and CXCL11 were all significantly elevated compared with SCT patients who did not develop cGVHD (Figure 1A 3-fold, 2-fold, and 2.5-fold, respectively, P < .05). In patients with cGVHD not involving the skin, no significant difference in the serum concentrations of CXCL9, CXCL10, and CXCL11 were identified (Figure 1A). Elevations of CXCL9, CXCL10, and CXCL11 were also identified when patients with cGVHD of the skin (n = 8) were compared with either HD controls (n = 6) or SCT controls (n = 18; Figure 1B). We note that all 3 chemokine serum levels were elevated in cGVHD irrespective of the control used. The levels of all 3 chemokines were not influenced by the type of transplantation received or the donor type (supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
CXCR3 chemokine ligands are elevated in patients with cGVHD. (A-B) Luminex analysis of serum levels of the CXCR3 chemokine ligands CXCL9, CXCL10, and CXCL11 in control SCT patients (n = 18), in SCT patients with skin disease only (n = 8) or with no skin involvement (n = 20; A) and in HD controls (n = 6), SCT controls (n = 18), and SCT patients with skin only GVHD (n = 8; B). Data are illustrated as log10 serum concentration in pg/mL, illustrating mean expression levels plus SEM. Data were confirmed as log normally distributed by pp-plot. *P < .05, **P < .005, and ***P < .0005 by independent t test.
CXCR3 chemokine ligands are elevated in patients with cGVHD. (A-B) Luminex analysis of serum levels of the CXCR3 chemokine ligands CXCL9, CXCL10, and CXCL11 in control SCT patients (n = 18), in SCT patients with skin disease only (n = 8) or with no skin involvement (n = 20; A) and in HD controls (n = 6), SCT controls (n = 18), and SCT patients with skin only GVHD (n = 8; B). Data are illustrated as log10 serum concentration in pg/mL, illustrating mean expression levels plus SEM. Data were confirmed as log normally distributed by pp-plot. *P < .05, **P < .005, and ***P < .0005 by independent t test.
Late/delayed aGVHD is a relatively new clinical entity defined empirically in the NIH consensus criteria. We investigated the relationship between this new entity and classic cGVHD in terms of the serum levels of CXCL9, CXCL10, and CXCL11. No significant differences were identified in serum levels of each of these 3 ligands in patients classified as delayed aGVHD versus classic cGVHD in our patient cohort (supplemental Figure 1B), with skin-only serum levels of all 3 cytokines being comparable between both delayed aGVHD and classic cGVHD (supplemental Figure 1C). We conclude that the chemokine ligand repertoires for CXCL9, CXCL10, and CXCL11 associated with both delayed aGVHD and classic cGVHD are the same, suggesting that these 3 cytokines have a mechanistic role in both forms of GVHD.
CD4+ T cells expressing CXCR3 are reduced in the peripheral blood of patients with cGVHD.
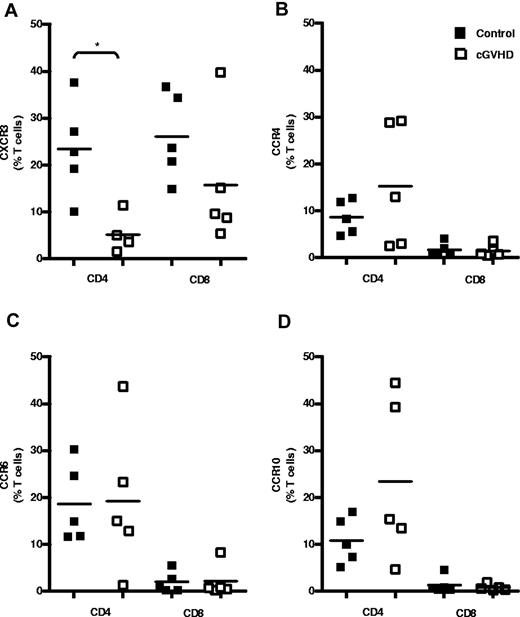
Because CXCL9, CXCL10, and CXCL11 levels were found to be increased in the serum of patients with cGVHD of the skin, we hypothesized that T cells expressing CXCR3, the receptor for these chemokines, are recruited to tissues with active cGVHD. The levels of CXCR3, CCR4, CCR6, and CCR10 expression on CD4+ and CD8+ T cells within the peripheral blood was therefore examined in SCT patients with or without cGVHD (Figure 2). Only 5% of CD4+ T cells expressed CXCR3+ in patients with cGVHD compared with a mean of 23% in the control group. The proportion of these cells was therefore reduced by nearly 80% in the GVHD group (n = 5, P = .016; Figure 2A). The number of CXCR3-expressing CD8+ T cells also showed a modest, but nonsignificant, reduction from 26% to 15% of CD8+ T cells in the patient group (n = 5; Figure 2A). In contrast, the expression of CCR4, CCR6, and CCR10 on CD4+ and CD8+ T cells was not altered in patients with GVHD compared with the control group (Figure 2B-D). No differences in the mean fluorescence intensity of each of the chemokine receptors was observed on either CD4+ or CD8+ T cells when SCT controls and patients with skin cGVHD were compared (supplemental Figure 2A-B).
Peripheral CD4+ T cells expressing CXCR3 are reduced in cGVHD. Shown is T-cell chemokine receptor expression in the peripheral blood of patients after allogeneic SCT. Shown are the results of flow cytometric analysis of cell-surface expression of CXCR3-PE (A), CCR4-PE-Cy7 (B), CCR6-PerCPCy5.5 (C), and CCR10-APC (D) on CD4+ and CD8+ T cells in control SCT patients (n = 5) and in SCT patients with cGVHD (n = 5). Data are shown as the frequency of total peripheral CD4+ and CD8+ T cells expressing each receptor in individual donors, with bars indicating mean expression levels for each group. *P < .05 by the Mann-Whitney U test.
Peripheral CD4+ T cells expressing CXCR3 are reduced in cGVHD. Shown is T-cell chemokine receptor expression in the peripheral blood of patients after allogeneic SCT. Shown are the results of flow cytometric analysis of cell-surface expression of CXCR3-PE (A), CCR4-PE-Cy7 (B), CCR6-PerCPCy5.5 (C), and CCR10-APC (D) on CD4+ and CD8+ T cells in control SCT patients (n = 5) and in SCT patients with cGVHD (n = 5). Data are shown as the frequency of total peripheral CD4+ and CD8+ T cells expressing each receptor in individual donors, with bars indicating mean expression levels for each group. *P < .05 by the Mann-Whitney U test.
Reduction in peripheral blood CXCR3+ CD4+ T cells observed during cGVHD is because of alteration in the proportion of CD4+ EM cells
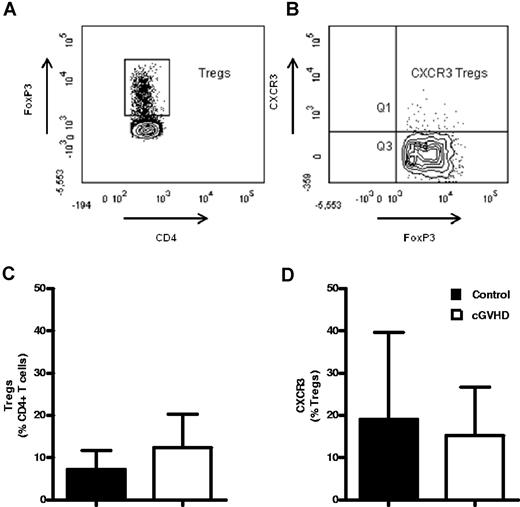
CD4+ Tregs may express CXCR3+, so it became important to clarify whether the reduction in CXCR3+ T cells that we had observed in the blood of patients with GVHD reflected alterations in the effector memory (EM) or Treg cell populations. Intracellular FoxP3 expression was therefore added to the flow cytometric panel to determine both the proportion of Treg cells in blood and the relative expression of CXCR3 on this subset (Figure 3A-B). The frequency of FoxP3+ Tregs was highly variable in both patients and controls, but no differences were observed between the 2 groups. Expression of CXCR3 on Treg populations also varied between 2% and 40%, but, again, was similar in the 2 groups. These data show that the reduction in CXCR3+ T cells that we observed within the blood of patients with GVHD was due to a decrease in the FoxP3− EM subset rather than a Treg population (Figure 3A-D).
The percentage and CXCR3 phenotype of peripheral blood FoxP3+ Tregs is not altered in patients with cGVHD. Shown is CXCR3 expression by regulatory CD4+ T cells in the peripheral blood of patients after allogeneic SCT. Shown are the results of representative flow cytometric staining of FoxP3+ CD4+ Tregs (n = 5; A) and CXCR3+ Tregs (n = 5; B). Peripheral blood was analyzed for surface expression of CD3-APC-Cy7 and CD4-FITC and for intracellular FoxP3-PE to determine frequencies of CD4+ Tregs, and for their surface expression of the chemokine receptor CXCR3-PerCPCy5.5 (n = 5). Figures show the mean frequency of CD4+ FoxP3+ Tregs (C) and of CXCR3-expressing Tregs in control SCT patients and in SCT patients with cGVHD (D). Data are shown as the mean frequency of CD4+ T cells expressing FoxP3 and of FoxP3+ cells expressing CXCR3. Bars indicate SEM. *P < .05 by the Mann-Whitney U test.
The percentage and CXCR3 phenotype of peripheral blood FoxP3+ Tregs is not altered in patients with cGVHD. Shown is CXCR3 expression by regulatory CD4+ T cells in the peripheral blood of patients after allogeneic SCT. Shown are the results of representative flow cytometric staining of FoxP3+ CD4+ Tregs (n = 5; A) and CXCR3+ Tregs (n = 5; B). Peripheral blood was analyzed for surface expression of CD3-APC-Cy7 and CD4-FITC and for intracellular FoxP3-PE to determine frequencies of CD4+ Tregs, and for their surface expression of the chemokine receptor CXCR3-PerCPCy5.5 (n = 5). Figures show the mean frequency of CD4+ FoxP3+ Tregs (C) and of CXCR3-expressing Tregs in control SCT patients and in SCT patients with cGVHD (D). Data are shown as the mean frequency of CD4+ T cells expressing FoxP3 and of FoxP3+ cells expressing CXCR3. Bars indicate SEM. *P < .05 by the Mann-Whitney U test.
Skin biopsies from patients with cGVHD contain an increased proportion of CD4+ and CXCR3+ T cells with an EM phenotype
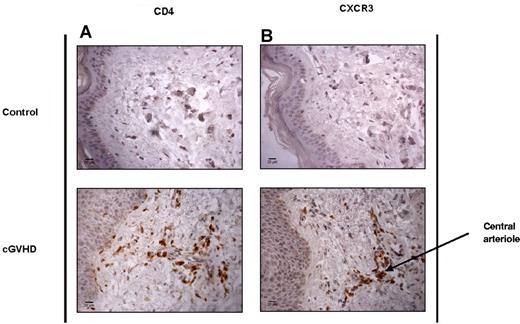
To determine whether CXCR3 expression was involved in the migration of T cells from peripheral blood into tissue during GVHD, we obtained fresh 4-mm skin-punch biopsies (Table 2) and paraffin-embedded tissue sections from patients with GVHD of the skin. Immunohistochemical analysis of paraffin-embedded tissue sections from HD controls and patients with cGVHD confirmed that infiltration of both CD4 and CXCR3+ T cells into the skin dermis was a specific feature of cGVHD (Figure 4A-B). These populations were often identified in clusters around the central arterioles of the dermis, which is likely to reflect their recent entry into the tissue.
Patient characteristics for fresh skin biopsy analysis including aGVHD and cGVHD SCT patients
| Group . | Patient ID . | Disease . | Transplantation date . | Time after transplantation . | Conditioning . | Transplantation type . | Alem . | GVHD prophylaxis . | Stem cell source . | NIH classification (retrospective) . | Previous aGVHD . | Grade . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| aGVHD | Biopsy A | MDS | 06-May-09 | 4 wk | Cy/TBI | MUD | In vivo | CSA + MTX | PBSCs | Classic aGVHD | N/A | N/A |
| Biopsy B | MDS | 23-Apr-09 | 10 wk | Flu/Mel/Alem, no TBI | SIB | In vivo | CSA only | PBSCs | Classic aGVHD | N/A | N/A | |
| Biopsy C | CML | 25-Aug-09 | 4 wk | Flu/Bu/Alem | SIB | In vivo | CSA only | PBSCs | Classic aGVHD | N/A | N/A | |
| Biopsy D | MDS | 06-Oct-09 | 3 wk | Flu/Mel/Alem, no TBI | SIB | In vivo | CSA only | PBSCs | Classic aGVHD | N/A | N/A | |
| Biopsy E | MDS | 22-Sep-09 | 11 wk | Flu/Mel/Alem, no TBI | MUD | In vivo | CSA only | PBSCs | Classic aGVHD | N/A | N/A | |
| Biopsy F | NHL | 20-Apr-10 | 11 wk | Flu/Mel/Alem, no TBI | MUD | In vivo | CSA only | PBSCs | Classic aGVHD | N/A | N/A | |
| Biopsy G | B-ALL | 02-Jun-10 | 7 wk | Cy/TBI | SIB | None | CSA + MTX | PBSCs | Classic aGVHD | N/A | N/A | |
| Biopsy H | MDS | 29-Jun-10 | 3 wk | Flu/Mel/Alem, no TBI | MUD | In vivo | CSA only | PBSCs | Classic aGVHD | N/A | N/A | |
| Biopsy I | CML | 12-Aug-10 | 6 wk | Flu/Bu/Alem | MUD | In vivo | CSA only | PBSCs | Classic aGVHD | N/A | N/A | |
| Biopsy J | AML | 16-Nov-10 | 4 wk | Flu/Mel/Alem, no TBI | MUD | In vivo | CSA only | PBSCs | Classic aGVHD | N/A | N/A | |
| cGVHD | Biopsy K | MF | 28-Apr-09 | 33 wk | Flu/Mel/Alem, no TBI | MUD | In vivo | CSA only | PBSCs | Late aGVHD | Skin | Grade I |
| Biopsy L | AML | 07-Apr-10 | 25 wk | Flu/Mel/Alem, no TBI | MUD | In vivo | CSA only | PBSCs | Classic cGVHD | None | N/A | |
| Biopsy M | AML | 14-Apr-10 | 22 wk | Flu/Mel/Alem, no TBI | MUD | In vivo | CSA only | PBSCs | Late aGVHD | Skin | Grade II | |
| Biopsy N | CLL | 20-Oct-09 | 47 wk | Flu/Mel/Alem, no TBI | MUD | In vivo | CSA only | PBSCs | Classic cGVHD | None | N/A | |
| Biopsy O | AML | 27-Apr-10 | 23 wk | Flu/Mel/Alem, no TBI | MUD | In vivo | CSA only | PBSCs | Classic cGVHD | None | N/A | |
| Biopsy P | MDS | 06-Oct-09 | 66 wk | Flu/Mel/Alem, no TBI | SIB | In vivo | CSA only | PBSCs | Classic cGVHD | Skin | Grade II | |
| Biopsy Q | AML | 17-Aug-10 | 24 wk | Flu/Mel/Alem, no TBI | SIB | In vivo | CSA only | PBSCs | Classic cGVHD | Gut | Not known | |
| Biopsy R | TPL | 22-Jun-10 | 78 wk | Cy/TBI | SIB | None | CSA + MTX | PBSCs | Late aGVHD | Gut | Grade II | |
| Biopsy S | CML | 05-Oct-10 | 42 wk | Cy/TBI/Alem | MUD | In vivo | CSA + MTX | PBSCs | Classic cGVHD | Skin | Grade I |
| Group . | Patient ID . | Disease . | Transplantation date . | Time after transplantation . | Conditioning . | Transplantation type . | Alem . | GVHD prophylaxis . | Stem cell source . | NIH classification (retrospective) . | Previous aGVHD . | Grade . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| aGVHD | Biopsy A | MDS | 06-May-09 | 4 wk | Cy/TBI | MUD | In vivo | CSA + MTX | PBSCs | Classic aGVHD | N/A | N/A |
| Biopsy B | MDS | 23-Apr-09 | 10 wk | Flu/Mel/Alem, no TBI | SIB | In vivo | CSA only | PBSCs | Classic aGVHD | N/A | N/A | |
| Biopsy C | CML | 25-Aug-09 | 4 wk | Flu/Bu/Alem | SIB | In vivo | CSA only | PBSCs | Classic aGVHD | N/A | N/A | |
| Biopsy D | MDS | 06-Oct-09 | 3 wk | Flu/Mel/Alem, no TBI | SIB | In vivo | CSA only | PBSCs | Classic aGVHD | N/A | N/A | |
| Biopsy E | MDS | 22-Sep-09 | 11 wk | Flu/Mel/Alem, no TBI | MUD | In vivo | CSA only | PBSCs | Classic aGVHD | N/A | N/A | |
| Biopsy F | NHL | 20-Apr-10 | 11 wk | Flu/Mel/Alem, no TBI | MUD | In vivo | CSA only | PBSCs | Classic aGVHD | N/A | N/A | |
| Biopsy G | B-ALL | 02-Jun-10 | 7 wk | Cy/TBI | SIB | None | CSA + MTX | PBSCs | Classic aGVHD | N/A | N/A | |
| Biopsy H | MDS | 29-Jun-10 | 3 wk | Flu/Mel/Alem, no TBI | MUD | In vivo | CSA only | PBSCs | Classic aGVHD | N/A | N/A | |
| Biopsy I | CML | 12-Aug-10 | 6 wk | Flu/Bu/Alem | MUD | In vivo | CSA only | PBSCs | Classic aGVHD | N/A | N/A | |
| Biopsy J | AML | 16-Nov-10 | 4 wk | Flu/Mel/Alem, no TBI | MUD | In vivo | CSA only | PBSCs | Classic aGVHD | N/A | N/A | |
| cGVHD | Biopsy K | MF | 28-Apr-09 | 33 wk | Flu/Mel/Alem, no TBI | MUD | In vivo | CSA only | PBSCs | Late aGVHD | Skin | Grade I |
| Biopsy L | AML | 07-Apr-10 | 25 wk | Flu/Mel/Alem, no TBI | MUD | In vivo | CSA only | PBSCs | Classic cGVHD | None | N/A | |
| Biopsy M | AML | 14-Apr-10 | 22 wk | Flu/Mel/Alem, no TBI | MUD | In vivo | CSA only | PBSCs | Late aGVHD | Skin | Grade II | |
| Biopsy N | CLL | 20-Oct-09 | 47 wk | Flu/Mel/Alem, no TBI | MUD | In vivo | CSA only | PBSCs | Classic cGVHD | None | N/A | |
| Biopsy O | AML | 27-Apr-10 | 23 wk | Flu/Mel/Alem, no TBI | MUD | In vivo | CSA only | PBSCs | Classic cGVHD | None | N/A | |
| Biopsy P | MDS | 06-Oct-09 | 66 wk | Flu/Mel/Alem, no TBI | SIB | In vivo | CSA only | PBSCs | Classic cGVHD | Skin | Grade II | |
| Biopsy Q | AML | 17-Aug-10 | 24 wk | Flu/Mel/Alem, no TBI | SIB | In vivo | CSA only | PBSCs | Classic cGVHD | Gut | Not known | |
| Biopsy R | TPL | 22-Jun-10 | 78 wk | Cy/TBI | SIB | None | CSA + MTX | PBSCs | Late aGVHD | Gut | Grade II | |
| Biopsy S | CML | 05-Oct-10 | 42 wk | Cy/TBI/Alem | MUD | In vivo | CSA + MTX | PBSCs | Classic cGVHD | Skin | Grade I |
Shown are the clinical features of fresh skin biopsies received from patients with aGVHD and cGVHD after SCT.
MDS, myelodysplastic syndrome; CML, chronic myeloid leukemia; NHL, non-Hodgkin lymphoma; B-ALL, B-cell acute lymphoblastic leukemia; AML, acute myeloid leukemia; CLL, chronic lymphocytic leukemia; TPL, T-prolymphocytic leukemia; Cy, cyclophosphamide; TBI, total body irradiation; Flu, fludarabine; Mel, melphalan; Alem, alemtuzumab; Bu, busulfan; MUD, matched unrelated donor; SIB, sibling donor; MTX, methotrexate; MF, myelofibrosis; and PBSCs, peripheral blood stem cells.
CD4+ and CXCR3+ T cells are elevated in the skin of SCT patients with cGVHD. Shown are the results of immunohistochemical analysis of CD4 and CXCR3 tissue distribution in control healthy skin and in SCT patients with cGVHD. Paraffin-embedded, antigen-retrieved tissue sections were incubated with CD4 (A) and CXCR3 (B) mAbs plus secondary anti–mouse/rabbit HRP. Staining was developed with DAB chromogen and sections counterstained with hematoxylin. Images were taken at room temperature on a Zeiss Axioscop 40 microscope with a Zeiss AxioCam MRc5 camera, magnification 40×/0.65 air. Images were acquired with AxioCam Version 4.4 software, and analyzed using ImageJ Version 1.46 software.
CD4+ and CXCR3+ T cells are elevated in the skin of SCT patients with cGVHD. Shown are the results of immunohistochemical analysis of CD4 and CXCR3 tissue distribution in control healthy skin and in SCT patients with cGVHD. Paraffin-embedded, antigen-retrieved tissue sections were incubated with CD4 (A) and CXCR3 (B) mAbs plus secondary anti–mouse/rabbit HRP. Staining was developed with DAB chromogen and sections counterstained with hematoxylin. Images were taken at room temperature on a Zeiss Axioscop 40 microscope with a Zeiss AxioCam MRc5 camera, magnification 40×/0.65 air. Images were acquired with AxioCam Version 4.4 software, and analyzed using ImageJ Version 1.46 software.
After digestion with collagenase D and removal of plastic-adherent cells, mononuclear cell populations were then also isolated from fresh 4-mm skin-punch biopsies from patients with cGVHD and compared with HD controls. This allowed specific flow cytometric analysis of dermal derived T-cell infiltrates. The expression of the chemokine receptors CCR4, CCR6, CCR7, CCR10, and CXCR3 was examined on CD3+ T cells with subdivision into CD45RA-expressing subsets (Figure 5A-D).
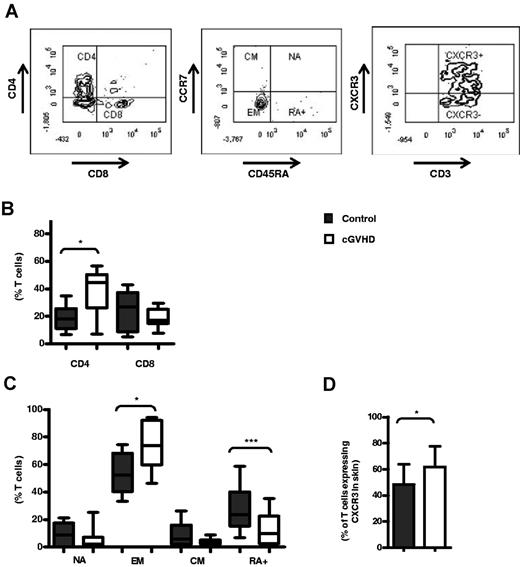
CD4+, CXCR3+, and EM cells are elevated in the skin of SCT patients with cGVHD. Shown are the results of flow cytometric analysis of fresh lymphocytes isolated from 4-mm skin-punch biopsies (A) Representative surface expression of CD4-ECD and CD8-AmCyan, CD45RA-AF700 and CCR7-FITC, and CXCR3-PE on CD3-APC-Cy7+ T cells. (B-C) CD4 and CD8 (B), naive (NA, CD45RA+CCR7+), EM (CD45RA−CCR7+), central memory (CM, CD45RA−CCR7+), and EM RA+ (RA+CD45RA+CCR7−) expression (C), and CXCR3+ expression (D) by CD3+ T cells in control skin (n = 10) and in patients with cGVHD (n = 9). Data are expressed as the median frequency of CD3+ T cells with bars illustrating the interquartile range (B-C) and as the mean frequency with bars illustrating SD (D). *P < .05 and **P < .005 by the Mann-Whitney U test; elevated CXCR3+ expression was confirmed by 1-way t test.
CD4+, CXCR3+, and EM cells are elevated in the skin of SCT patients with cGVHD. Shown are the results of flow cytometric analysis of fresh lymphocytes isolated from 4-mm skin-punch biopsies (A) Representative surface expression of CD4-ECD and CD8-AmCyan, CD45RA-AF700 and CCR7-FITC, and CXCR3-PE on CD3-APC-Cy7+ T cells. (B-C) CD4 and CD8 (B), naive (NA, CD45RA+CCR7+), EM (CD45RA−CCR7+), central memory (CM, CD45RA−CCR7+), and EM RA+ (RA+CD45RA+CCR7−) expression (C), and CXCR3+ expression (D) by CD3+ T cells in control skin (n = 10) and in patients with cGVHD (n = 9). Data are expressed as the median frequency of CD3+ T cells with bars illustrating the interquartile range (B-C) and as the mean frequency with bars illustrating SD (D). *P < .05 and **P < .005 by the Mann-Whitney U test; elevated CXCR3+ expression was confirmed by 1-way t test.
CD4+ T cells represented a median of 18% of the CD3+ population in skin biopsies from the control group, but were increased to 44% in patients with cGVHD (n = 10 controls, n = 9 cGVHD patients; Figure 5B). The percentage of CCR7−CD45RA− EM T cells was also elevated from 52% of the T-cell pool in control samples to 74% in patients with cGVHD (P = .028). In association with this, a reduction in the number of naive, central memory, and CD45RA+ “revertant” memory cells (P = .0006) was observed in the patient group (Figure 5C). The percentage of CXCR3+ expression on T cells within skin was 48% in the control group, but increased to 62% in GVHD biopsies (n = 11 control, n = 10 GVHD, P = .03; Figure 5D). The frequencies of naive, central memory, EM, RA+, and CXCR3+ cells observed within the CD3+, CD4+, and CD8+ population of cGVHD patients were equivalent, with a trend observed toward a greater frequency of EM cells in both the CD4+ and CD8+ groups (supplemental Figure 3A-B). Consistent with our observations in relation to PBMCs, no changes in T-cell expression of CCR4, CCR6, or CCR10 were observed in the patient cohort (supplemental Figure 3C).
Relative proportion of CD4+ T cells helps to distinguish between classic aGVHD and skin cGVHD
We and others have previously reported that CXCL10 levels are increased in patients with aGVHD and that this is associated with the migration of CXCR3+ T cells into affected tissue.15 To compare the pattern of T-cell infiltration associated with these 2 major subtypes of disease directly, we determined the relative frequencies of CD4+, CD8+, naïve, and memory T cells isolated from skin biopsies of aGVHD (n = 10) and cGVHD (n = 9) patients. The chemokine receptor expression on these populations was also determined. No differences were observed in the proportion of CD8+ T cells (Figure 6A), naive, and memory subsets (Figure 6B) or global chemokine receptor expression levels between the 2 groups (Figure 6C and supplemental Figure 3C). However, a striking increase in the frequency of CD4+ T cells was observed in patients with the late form of disease (Figure 6A). CD4+ T cells represented a median of 44.6% in patients with late GVHD compared with only 12.7% in the aGVHD group (P < .05). This finding supports the concept that CD4+ T cells may play a predominant role in the pathogenesis of cGVHD, and this observation may be of clinical value in helping to differentiate at a pathologic level between classic aGVHD and cGVHD.
Higher CD4+ T-cell frequencies are observed in cGVHD but not skin aGVHD. Flow cytometric analysis of fresh lymphocytes isolated from 4-mm punch-skin biopsies after collagenase digestion. Shown is a comparison of CD4 (ECD) and CD8 (AmCyan) frequencies (A), naive (NA, CD45RA+, AF700), CCR7+ (FITC), EM (CD45RA−CCR7−), central memory (CM, CD45RA−CCR7+), and EM RA+ (RA+CD45RA+CCR7−) frequency (B), and CXCR3+(PE) expression (C) by CD3+ T cells in the skin of patients with aGVHD (n = 10) and cGVHD (n = 9). Data are expressed as the median frequency of CD3+ T cells with bars illustrating the interquartile range (A-B) and as the mean frequency with bars illustrating SEM (C-D). *P < .05 and **P < .005 by the Mann-Whitney U test.
Higher CD4+ T-cell frequencies are observed in cGVHD but not skin aGVHD. Flow cytometric analysis of fresh lymphocytes isolated from 4-mm punch-skin biopsies after collagenase digestion. Shown is a comparison of CD4 (ECD) and CD8 (AmCyan) frequencies (A), naive (NA, CD45RA+, AF700), CCR7+ (FITC), EM (CD45RA−CCR7−), central memory (CM, CD45RA−CCR7+), and EM RA+ (RA+CD45RA+CCR7−) frequency (B), and CXCR3+(PE) expression (C) by CD3+ T cells in the skin of patients with aGVHD (n = 10) and cGVHD (n = 9). Data are expressed as the median frequency of CD3+ T cells with bars illustrating the interquartile range (A-B) and as the mean frequency with bars illustrating SEM (C-D). *P < .05 and **P < .005 by the Mann-Whitney U test.
Discussion
cGVHD represents a major clinical challenge in the development of SCT with a pathogenesis that is poorly understood. Many clinical features resemble disorders such as scleroderma or systemic lupus erythematosus, and this has led to suggestions that both allo-immune and auto-immune T-cell recognition may contribute to the disease pathogenesis.20-23
In the present study, we found that the IFNγ-inducible chemokines CXCL9, CXCL10, and CXCL11 are increased by up to 3-fold in the serum of patients with cGVHD of the skin. This provides an insight into the pathogenesis of cGVHD, for which there is a paucity of studies investigating the mechanism of this devastating condition.15 In contrast to aGVHD, in which CXCL10 was the only one of the CXCL9-CXCL11 family members to show an increase,15 in the present study, we discovered elevations of all 3 CXCR3 ligands in patients with skin cGVHD. The relative individual physiologic roles of the CXCR3-binding chemokines are uncertain, but these observations suggest that CXCL10, the most dominant of the CXCR3 ligands, may be the first to increase during the development of aGVHD and is then followed by a broadening of chemokine expression as the disease progresses to include CXCL9 and CXCL11. The same pattern of chemokine ligands was identified when comparing patients with skin cGVHD with SCT patients without cGVHD and also with HD controls. There were no differences observed in serum levels of CXCL9, CXCL10, or CXCL11 in patients with delayed aGVHD and classic cGVHD, suggesting that the empirical clinical distinction between these 2 entities may not reflect a genuine difference in pathogenesis. cGVHD is more common in patients who have suffered from the acute form of the disease, so it is possible that the high levels of CXCL10 that we observed simply reflect a residual elevation in this subgroup. However, arguing against this is the fact that CXCL10 levels generally return to normal within 100 days of an episode of aGVHD,15 whereas many patients herein were studied at time points up to 2 years after transplantation. Therefore, these observations suggest that CXCR3-mediated interactions do indeed have a specific role in cGVHD.
To consider the impact of factors that have previously been shown to influence GVHD, we considered the potential influence of transplantation type, donor type, and prophylaxis on GVHD incidence. This study was not powered to determine a difference between those receiving reduced intensity and those receiving myeloablative conditioning or between sibling and matched unrelated donor transplantations in regard to chemokine ligand expression. Given that caveat, no difference was found when these groups were analyzed.
It is also important to consider the possible effects of GVHD prophylaxis and treatment in relation to serum levels of CXCL9, CXCL10, and CXCL11. After transplantation, patients received either CSA with in vivo T-cell depletion using alemtuzumab or short-course methotrexate plus CSA as GVHD prophylaxis. Patients with limited skin disease received topical corticosteroid therapy, with systemic corticosteroids or calcineurin inhibitors used for moderate or severe disease. In the event of refractory or recurrent cGVHD, patients are eligible for electrocorporeal photophoresis, and if they fail that treatment or have symptoms in addition to mucocutaneous cGVHD, they receive mycophenolate mofetil, rituximab, or imatinib. Individual treatment strategies are listed in Table 1 for each patient described. Effects of such therapeutics as infliximab and CSA on the production of CXCL10 reveal reduced levels of chemokines in response to IFNγ and TNFα treatment,24 and reduced levels of chemokines are also found in response to oral and inhaled corticosteroids in chronic obstructive pulmonary disease.25 Therefore, in patients in whom treatment was ongoing before sample collection, the levels of chemokines detected may be lower than would have been identified at the time of initial presentation.
CXCL10-mediated migration of CXCR3+ T cells seems to play an important role in the provenance of both aGVHD and cGVHD of the skin. CXCL10 expression is also elevated in the early stage of systemic sclerosis, which shares many clinical features of cGVHD,26 as well as in oral periapical disease,27 and CXCR3 has been shown to play a critical role in the development of renal Th1 and Th17 immune responses in murine lupus nephritis.28 Several recent studies also support a role for CXCL9, CXCL10, and CXCL11 cGVHD. Elevated expression of these chemokine genes was observed in a murine model of sclerodermatous GVHD,29 and the accumulation of T-bet+ EM cells was observed in the oral mucosa of patients in association with increased expression of CXCL9.16 CXLC10 and CXCL11 can both be produced locally in the skin and ocular tissue by fibroblasts on activation with proinflammatory cytokines including TNFα and IL-4, so it is possible that local production of CXCL9, CXCL10, and CXCL11 plays a critical role in the early stages of the cGVHD process.30 Further fibroblast activation after local immune-mediated inflammation may lead to additional chemokine production, thus setting up a positive feedback cycle. Activated fibroblasts can express HLA-DR and CD40 in response to IFNγ,31 and therefore may provide a source of antigen presentation to local CD4+ T cells.
The common receptor for CXCL9, CXCL10, and CXCL11 is CXCR3, and involvement of CXCR3+ T cells at disease sites has been observed in cGVHD.16,17 To analyze changes in CXCR3, in the present study, we analyzed CXCR3+ expression in both the peripheral blood and in the skin. Because ethics considerations do not allow biopsies to be taken from patients without symptoms of GVHD because of their increased susceptibility to infections, HD volunteers were used as controls. However, because the same changes in chemokine ligands were observed comparing GVHD patients with HD controls or SCT controls (Figure 1B), we conclude that this is an appropriate control for such experiments.
We noted a dramatic reduction in the proportion of CD4+ T cells that express CXCR3+ within peripheral blood at the time of cGVHD of the skin. This was associated with an accumulation of both CD4+ and CXCR3+ cells within skin biopsies, as shown by immunohistochemistry and FACS analysis of intradermal T cells. Because subpopulations of FoxP3+ Tregs may express CXCR3, it was thought possible that these observed changes in tissue distribution of CD4+CXCR3+ cells might reflect alterations in the proportion of this cell subset. However, we did not observe an alteration in either the proportion of these cells in the peripheral blood or in their relative expression of CXCR3 in the patient or control groups, indicating that differential migration was a reflection of alterations in the EM subpopulations. No changes in the mean fluorescence intensity of chemokine receptors were observed in control or cGVHD patients, indicating that the changes in the frequency of cells was due to increased movement toward the tissues, rather than to the receptor internalization that may occur after ligand interaction. However, this cannot be fully confirmed and quantitative PCR analysis would be required in this context.
A striking feature of our present results was the predominance of CD4+ T cells in skin biopsies taken from patients with late-onset disease. Although animal models of cGVHD are difficult to establish, a unifying observation has been that tissue damage is mediated primarily by CD4+ T cells. A model of sclerodermatous GVHD demonstrated CD4+ T-cell activation to be a key feature of the disease process accompanied by strong recruitment of cells to the skin.5 The critical role of STAT3 signaling in murine cGVHD also indicates the central role for Th1 CD4 T cells and the importance of the balance between EM cells and Tregs.32 It remains unclear if this CD4+ T-cell response derives from mature donor cells introduced at the time of the transplantation or if it follows maturation of hematopoietic stem cell–derived CD4+ T cells in the host thymus. The latter model certainly plays a primary role in some situations and has led to efforts to reduce thymic damage in an effort to limit the development of nontolerant CD4+ T cells.6 Our present results demonstrate the involvement of CD4+ EM T cells in human delayed-onset disease and significantly differentiates the disease process from that mediated in classic aGVHD, in which CD4+ T cells represent only a minor subpopulation of the T-cell infiltrate. A conventional view has been that murine CD4+ EM T cells mediate tissue damage primarily through noncytotoxic effector mechanisms, with high levels of local IFNγ being a feature of the disease.4,5 The exact mechanisms of tissue damage in human cGVHD are uncertain, but may relate directly to cytokine-mediated effects, activation of cytotoxic alloreactive CD8 cells, or support for B-cell survival. Indeed, B cells have been implicated in the pathogenesis of cGVHD (for review, see Kapur et al33 ), and a recent study using rituximab in steroid-refractory cGVHD led to a clinical response in 70% of subjects.34 CD4+ T cells have been shown to reconstitute at a slower rate than CD8+ T cells after SCT,35 so late-onset delayed aGVHD and classic cGVHD may manifest at this later time point because of the requirement for CD4 help. Natural killer cells have themselves been shown to play an important role in limiting alloreactive CD4+ T-cell expansion in cGVHD,36 and it will be important in future studies to investigate the relative numbers and phenotype of cells of the innate immune system in the human form of the disease.
In summary, the results of the present study demonstrate that the production of CXCR3-binding cytokines leads to the recruitment of peripheral blood CXCR3+ and CD4+ T cells into disease sites in patients with cGVHD (Figure 7). An understanding of the chemotactic processes that direct T cells to the tissues in cGVHD will help to direct the introduction of chemokine blockade as a potential therapy to prevent or treat one of the most enduring problems of SCT. We are now prospectively exploring the temporal dynamics of ligand concentration to define the time at which chemokine blockade might be initiated within individual patients.
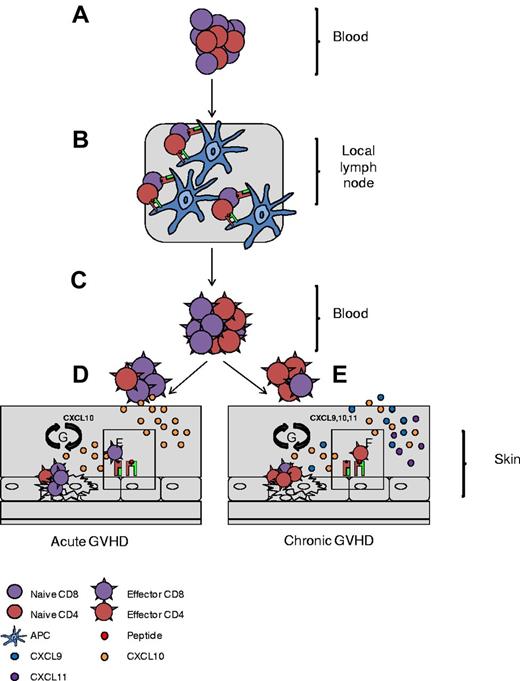
Schematic representation of CD4+ T-cell involvement in cGVHD pathogenesis. Naive T cells traffic to local lymph nodes (A), where they recognize recipient minor antigen (mAg) presented by APCs (B). (C) Primed naive cells convert to mAg-specific effector cells and exit the lymph nodes. (D-E) In aGVHD, CD8+ effectors migrate along a CXCL10 chemokine gradient toward the skin (D) and in cGVHD, CD4+ effectors are preferentially recruited via CXCL9, CXCL10, and CXCL11 chemokine gradients (E). (F) On entrance into the skin, CD4/CD8+ T cells recognize recipient mAg expressed on MHC I and MHC II, respectively. (G) Effectors then direct tissue-specific killing, promoting local cytokine and chemokine production and setting up a cycle of tissue destruction and further effector recruitment.
Schematic representation of CD4+ T-cell involvement in cGVHD pathogenesis. Naive T cells traffic to local lymph nodes (A), where they recognize recipient minor antigen (mAg) presented by APCs (B). (C) Primed naive cells convert to mAg-specific effector cells and exit the lymph nodes. (D-E) In aGVHD, CD8+ effectors migrate along a CXCL10 chemokine gradient toward the skin (D) and in cGVHD, CD4+ effectors are preferentially recruited via CXCL9, CXCL10, and CXCL11 chemokine gradients (E). (F) On entrance into the skin, CD4/CD8+ T cells recognize recipient mAg expressed on MHC I and MHC II, respectively. (G) Effectors then direct tissue-specific killing, promoting local cytokine and chemokine production and setting up a cycle of tissue destruction and further effector recruitment.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Alison Stewart for help with valuable patient information and Dr Claudia Roberts for supplying patient samples for immunohistochemical analysis.
This work was supported by the Leukemia & Lymphoma Society (research grants 08023 and 09023). Financial support for J.E.C was provided by the Stem Cell Transplant Trust Fund at University Hospital Birmingham (Birmingham, United Kingdom).
Authorship
Contribution: J.E.C. and P.A.H.M. designed and performed the experiments, analyzed and interpreted the data, performed the statistical analysis, and wrote the manuscript; C.F.I. performed the statistical analysis and wrote the manuscript; B.E.A. performed the experiments; S.N. and J.N. recruited the patients and provided clinical materials and information; and P.M., C.C, R.M., and P.A.H.M. provided clinical information on patients and wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Dr Joanne Elizabeth Croudace, School of Cancer Sciences, University of Birmingham, Vincent Drive, Edgbaston, B152TT United Kingdom; e-mail: j.e.croudace@bham.ac.uk.
References
Author notes
R.M. and P.A.H.M. contributed equally to this work.


This feature is available to Subscribers Only
Sign In or Create an Account Close Modal