In this issue of Blood, Schwamb and colleagues describe a novel mechanism of action through which kinase inhibitors can overcome B-cell receptor (BCR)–mediated survival of chronic lymphocytic leukemia (CLL) cells via their influence on sphingolipid metabolism.1
Management and outcome of patients with CLL have changed during the past decade due to improvements in molecular diagnostics and the implementation of targeted drug therapy.2,3 This rapid progress was due to the discovery of chromosomal changes, the understanding of the prognostic impact of the mutational status of immunoglobulin heavy chain genes, the discovery of prognostic markers and potential therapeutic targets by gene expression profiling, and recently by sequence analysis of the CLL genome.4 We have learned that CLL cell survival is strongly influenced by interaction with the microenvironment through cell-to-cell contact or via soluble factors and surface receptors. The central molecule in this cross-talk is the BCR composed of immunoglobulin heavy and light chains.5 The BCR ensures survival of B-cells by transduction of external stimuli to the inner cell through an elaborate signaling pathway (see figure). BCR-related genes and proteins are constantly stimulated in bone marrow and lymph nodes of patients with CLL.6
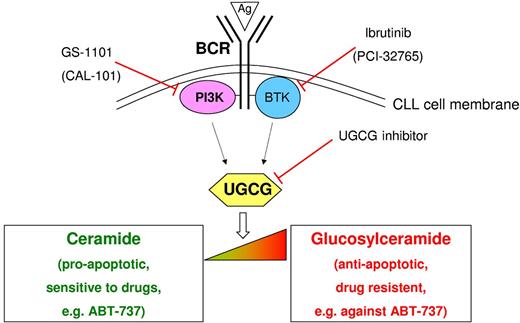
Modulation of the ceramide:glucosylceramide equilibrium by B-cell receptor (BCR) signaling and the novel kinase inhibitors GS-1101 and ibrutinib in CLL. BCR stimulation by antigens (Ags) or anti-IgM results in a shift from ceramide (proapoptotic) to glucosylceramide (antiapoptotic) mediated by up-regulation of UDP-glucose ceramide glucosyltransferase (UGCG). Direct inhibition of UGCG induces chronic lymphocytic leukemia (CLL) cell apoptosis. The kinase-specific inhibitors GS-1101 (PI3K) and ibrutinib (BTK) also cause down-regulation of UGCG leading to restoration of the δ ceramide:glucosylceramide equilibrium and leukemic cell death. This constitutes a novel mechanism of drug action. In addition, this effect on sphingolipid metabolism sensitizes the cells for mitochondria-targeting drugs such as ABT-737. Figure adapted from Schwamb et al; see Figure 6 in the article that begins on page 3978.
Modulation of the ceramide:glucosylceramide equilibrium by B-cell receptor (BCR) signaling and the novel kinase inhibitors GS-1101 and ibrutinib in CLL. BCR stimulation by antigens (Ags) or anti-IgM results in a shift from ceramide (proapoptotic) to glucosylceramide (antiapoptotic) mediated by up-regulation of UDP-glucose ceramide glucosyltransferase (UGCG). Direct inhibition of UGCG induces chronic lymphocytic leukemia (CLL) cell apoptosis. The kinase-specific inhibitors GS-1101 (PI3K) and ibrutinib (BTK) also cause down-regulation of UGCG leading to restoration of the δ ceramide:glucosylceramide equilibrium and leukemic cell death. This constitutes a novel mechanism of drug action. In addition, this effect on sphingolipid metabolism sensitizes the cells for mitochondria-targeting drugs such as ABT-737. Figure adapted from Schwamb et al; see Figure 6 in the article that begins on page 3978.
Contact of the BCR with an antigen (or in the experimental setting an anti-IgM antibody) triggers a cascade of events including calcium influx, concerted association of proteins at the intracellular BCR portion, protein phosphorylation, activation of transcription factors, and integrins. Constant antigenic stimulation of the BCR is thought to be driving leukemia development.7 BCR activation results in enhanced cell survival, proliferation, and cellular adhesion making this pathway an interesting target for antileukemic therapy. Specific inhibition of 2 kinases associated with BCR activation (phosphatidylinosytol-3-kinase 3, PI3K, and Bruton tyrosine kinase, BTK) has recently shown impressive clinical results.8,9
Changes in lipid metabolism associated with immunoglobulin mutation status have previously been observed.10 The novelty of the paper by Schamb et al lies in the description of the functional modulation of sphingolipid metabolism in CLL cells as a consequence of BCR stimulation, linking this process to potential therapeutic applications.1 The authors have investigated changes in the intracellular ratio between ceramide and glucosylceramide on BCR stimulation in primary CLL cells. The proapoptotic ceramide is converted to the antiapoptotic glucosylceramide by the enzyme UDP-glucose ceramide glucosyltransferase (UGCG; see figure). On BCR stimulation, UGCG expression is induced and the equilibrium shifted toward glucosylceramide leading to increased CLL cell survival. This survival effect is abrogated by direct inhibition of UCGC. Importantly, reduced expression of UGCG is also observed after treatment with the PI3K δ inhibitor CAL-101/GS-1101 or the BTK inhibitor ibrutinib (PCI-32765). It is unclear if this is a direct effect or mediated by kinase inhibition. Sphingolipids are also known as mediators of mitochondrial apoptosis. Consistent with the antiapoptotic effect of glucosylceramide, BCR stimulation reduced the effect of the mitochondria-targeting drug ABT-737. Concomitant treatment with low doses of the kinase (and apparently UGCG) inhibitors GS-1101 and ibrutinib restored the activity of ABT-737 leading to CLL cell death. Thus, the 2 drugs may also be regarded as sensitizers for ABT-737, opening up the possibility for novel drug combinations.
The discovery adds novel aspects to our understanding of BCR function as well as the potential implications for drug therapy. It will help to explain changes in (sphingo-) lipid metabolism as well as BCR-triggered gene regulation in CLL. This opens up a whole new approach for treatment options, the mechanism of action and functional downstream effects of novel kinase inhibitors as well as many other drugs that act on the BCR or one of its signaling pathway components. CLL therapy continues to be a success story.
Conflict-of-interest disclosure: The author declares no competing financial interests. ■

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal