To the editor:
We read with great interest the article by Kuo et al on a retrospective study of Helicobacter pylori (Hp) eradication as exclusive treatment in Taiwanese patients with early-stage gastric diffuse large B-cell lymphoma (DLBCL).1 Interestingly, a substantial portion of these aggressive lymphomas is responsive to antibiotics, but the authors recommend their results should be taken as investigational until validated by prospective studies. Recently, we concluded the first multicenter prospective trial addressing this issue in Western countries, aiming to demonstrate that a proportion of these patients can achieve long-term remission with antibiotics alone, leaving intact the probabilities of cure for unresponsive patients.
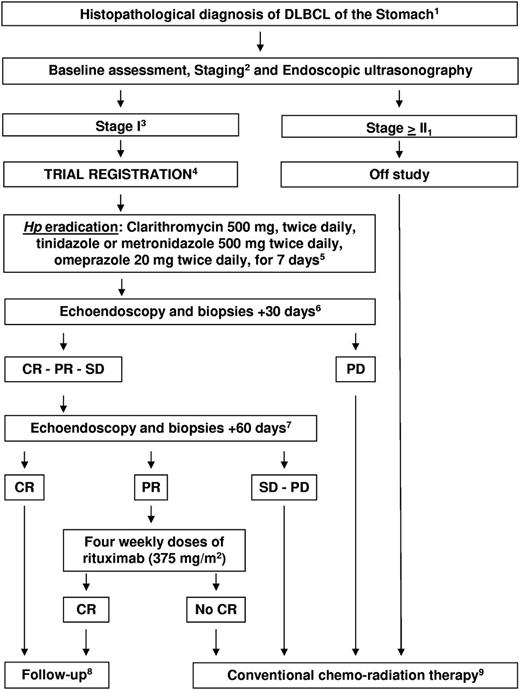
Sixteen patients with stage-I Hp-related gastric DLBCL, with (n = 5) or without (de novo; n = 11) MALT areas, were registered (supplemental Table 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). Antibiotic therapy included clarithromycin, tinidazole or metronidazole, and omeprazole (Figure 1). Hp eradication and tumor response were assessed at +30 and +60 days after antibiotics: patients achieving complete remission (CR) were referred to follow-up; patients with partial response (PR) received rituximab; and patients with stable/progressive disease received chemo-radiotherapy.
Study algorithm. 1Diagnostic biopsies underwent centrally pathology review.6 2Baseline and staging procedures: hemogram, biochemical profile, HIV, hepatitis B and C viruses' infection markers, contrasted thorax-abdomen CT scan, bone marrow biopsy, breath test, gastroscopy with at least 5 biopsies for each lesion and random sampling of residual normal mucosa, and echoendoscopy to evaluate gastric wall thickness and perigastric lymph nodes. The choice of areas suitable for biopsies was driven by echoendoscopy. Hp infection was confirmed by gastric biopsies ± breath test. 3Perigastric lymphadenopathies of a diameter < 1.5 cm were admitted to avoid a potential selection bias related to specificity limits of echoendoscopy. This cut-off was in line with the cut-off value used by standardized lymphoma criteria to define lymph-node infiltration when assessed by non-functional exams.7 4Sample size was not prospectively estimated in comparison with ORR reported with conventional chemo-radiotherapy (90%-100%) since it would request a non-inferiority design and hundreds of patients. Written informed consent was obtained from each registered patient; the trial conformed to the tenets of the Declaration of Helsinki and was approved by the IRB of participating centers. 5Patients who failed eradication received a second-line antibiotic therapy following local guidelines. 6The primary end point was overall response rate (ORR) after Hp eradication. Response was defined according to standardized criteria.7 Residual macroscopic abnormalities at endoscopic examination or residual perigastric lymph nodes measuring < 1 cm in diameter or gastric wall alterations at ultrasonography were considered as CR if histopathologic examination did not show lymphomatous infiltration. Persistence of areas of MALT and/or DLBCL in histopathologic specimens in patients with normal/improved gastric aspect at endoscopy and ultrasonography were considered as PR. 7Enhanced total-body CT scan was performed to exclude systemic dissemination. 8Physical examination, hemogram and biochemical profile, gastroscopy, gastric echoendoscopy, and enhanced CT scan every 3 months for the first 2 years, every 6 months from the 3rd to the 5th year, and once a year from the 6th to the 10th year. 9CHOP or CHOP-like regimens ± rituximab ± radiotherapy (physician's preference).
Study algorithm. 1Diagnostic biopsies underwent centrally pathology review.6 2Baseline and staging procedures: hemogram, biochemical profile, HIV, hepatitis B and C viruses' infection markers, contrasted thorax-abdomen CT scan, bone marrow biopsy, breath test, gastroscopy with at least 5 biopsies for each lesion and random sampling of residual normal mucosa, and echoendoscopy to evaluate gastric wall thickness and perigastric lymph nodes. The choice of areas suitable for biopsies was driven by echoendoscopy. Hp infection was confirmed by gastric biopsies ± breath test. 3Perigastric lymphadenopathies of a diameter < 1.5 cm were admitted to avoid a potential selection bias related to specificity limits of echoendoscopy. This cut-off was in line with the cut-off value used by standardized lymphoma criteria to define lymph-node infiltration when assessed by non-functional exams.7 4Sample size was not prospectively estimated in comparison with ORR reported with conventional chemo-radiotherapy (90%-100%) since it would request a non-inferiority design and hundreds of patients. Written informed consent was obtained from each registered patient; the trial conformed to the tenets of the Declaration of Helsinki and was approved by the IRB of participating centers. 5Patients who failed eradication received a second-line antibiotic therapy following local guidelines. 6The primary end point was overall response rate (ORR) after Hp eradication. Response was defined according to standardized criteria.7 Residual macroscopic abnormalities at endoscopic examination or residual perigastric lymph nodes measuring < 1 cm in diameter or gastric wall alterations at ultrasonography were considered as CR if histopathologic examination did not show lymphomatous infiltration. Persistence of areas of MALT and/or DLBCL in histopathologic specimens in patients with normal/improved gastric aspect at endoscopy and ultrasonography were considered as PR. 7Enhanced total-body CT scan was performed to exclude systemic dissemination. 8Physical examination, hemogram and biochemical profile, gastroscopy, gastric echoendoscopy, and enhanced CT scan every 3 months for the first 2 years, every 6 months from the 3rd to the 5th year, and once a year from the 6th to the 10th year. 9CHOP or CHOP-like regimens ± rituximab ± radiotherapy (physician's preference).
Results were excellent, and similar to those reported by Taiwanese colleagues (supplemental Table 2). All patients achieved Hp eradication; 1 patient required a second-line antibiotic therapy. Lymphoma response after eradication was CR in 8 (50%) patients, and PR in 3 (overall response rate = 69%; 95% confidence interval [CI] = 47%-91%; supplemental Figure 1); 5 patients had progressive disease. Two of the 3 PRs achieved CR after rituximab (supplemental Table 3). Thus, 10 patients achieved CR after antibiotics ± rituximab (complete response rate = 63%; 95% CI = 39%-87%).
Lymphoma regression was documented in all investigated subgroups, divided according to histologic subtype (MALT-related vs de novo DLBCL), ontogenic stratification2 (“germinal-center B cell–like” vs “nongerminal-center B cell–like” DLBCL), and presence of perigastric lymph nodes (yes vs no; supplemental Table 3). Regression of de novo DLBCL is an important achievement since a few responsive cases have been reported,3 whereas patients with de novo DLBCL were not assessed prospectively in the Kuo et al's study.1 Interpretation of these responses could be biased by the lack of recognition of MALT areas in scanty or undersized biopsies.4 However, the extensive bioptic mapping used in our trial bona fide rules out this bias and confirms that a proportion of de novo DLBCL are Hp-dependent. Responses in patients with perigastric lymphadenopathies are a challenging finding. Noteworthy, lymphadenopathies lacked confirmation of tumor infiltration both in this trial and in occasionally reported cases,5 whereas patients with stage II1 disease included in the Taiwanese study were not analyzed separately.1 Nevertheless, our results suggest that the presence of small perigastric lymphadenopathies is not a contraindication for exclusive treatment with antibiotics.
Two-thirds of patients achieved long-term remission without chemo-radiotherapy. At a median follow-up of 68 months (range 14-114), 9 of the 10 CRs remain relapse-free (median progression-free survival: 83+ months). Patients with unresponsive/relapsed lymphoma retained unaltered their probabilities of cure; all of them achieved CR after chemo-radiotherapy and remain relapse-free at 13-90 months (median 55+). No patient died of lymphoma; 14 patients are alive (5-year OS: 94%), and elderly patients died of cardiac failure and gallbladder cancer at 13 and 90 months, respectively.
Although encompassing small series and exhibiting some design differences, this trial and the Taiwanese study demonstrate that Hp eradication, keeping chemo-radiotherapy for unresponsive patients, is an affordable strategy for patients with limited-stage gastric DLBCL. An international trial aimed to extend these encouraging results and to distinguish the best candidates for this conservative strategy is warranted.
Authorship
The online version of this article contains a data supplement.
Acknowledgments: The authors are thankful to Dr Giuseppe Cannatelli (Pathology Unit, Azienda Ospedaliera di Crema, Crema, Italy), Dr Riccardo Valli (Pathology Unit, Ospedale Santa Maria Nuova, Reggio Emilia, Italy), Drs Marina Milani and Antonino Maiorana (Pathology Unit, Policlinico di Modena, Modena, Italy), Dr Aroldo Rizzo (Pathology Unit, Ospedale Cervello, Palermo, Italy), Dr Maria Teresa Enrica Martini (Pathology Unit, Ospedale Santo Spirito Regina Margherita, Rome, Italy), Dr Anna Guidetti (Oncologia Medica 3, Istituto Nazionale dei Tumori, Milan, Italy), Dr Andrea Carnevali (Pathology Unit, Azienda Ospedaliera di Arezzo, Arezzo, Italy), Dr Marcello Guarino (Pathology Unit, Azienda Ospedaliera di Vimercate, Vimercate, Italy), Dr Rosa Lotta (ISMETT, Palermo, Italy), and Dr Domenico Novero (Pathology Unit, Azienda Ospedaliera e Universitaria San Giovanni Battista, Turin, Italy) for their sustained scientific collaboration and for kindly providing clinical data and histopathologic material for analysis. They appreciate iconographic material kindly provided by Dr Angelo Zullo (Gastroenterologia ed Endoscopia Digestiva, PTP Nuovo Regina Margherita, Rome, Italy) and the excellent assistance of Dr Giuseppina Dognini (Unit of Lymphoid Malignancies, San Raffaele Scientific Institute, Milan, Italy), and Mrs Eliana di Cairano (Pathology Unit, San Raffaele Scientific Institute, Milan, Italy) in biologic material management and data collection.
Contribution: A.J.M.F., E.V., P.G.A., M.P., and C.P. designed research, performed analyses, and wrote the paper; S.G., M.R., A.M., A.A., S.D.O., D.C., L.D., F.I., and S.L. performed research, treated patients, and collected data; and M.P. and L.M. performed central pathology review and histopathologic investigations.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Andrés J. M. Ferreri, MD, Unit of Lymphoid Malignancies, Department of Oncohematology, San Raffaele Scientific Institute, Via Olgettina 60, 20132, Milan, Italy; e-mail: andres.ferreri@hsr.it.