Abstract
TGF-β activated kinase 1 (TAK1) is a mediator of various cytokine signaling pathways. Germline deficiency of Tak1 causes multiple abnormalities, including dilated blood vessels at midgestation. However, the mechanisms by which TAK1 regulates vessel formation have not been elucidated. TAK1 binding proteins 1 and 2 (TAB1 and TAB2) are activators of TAK1, but their roles in embryonic TAK1 signaling have not been determined. In the present study, we characterized mouse embryos harboring endothelial-specific deletions of Tak1, Tab1, or Tab2 and found that endothelial TAK1 and TAB2, but not TAB1, were critically involved in vascular formation. TAK1 deficiency in endothelial cells caused increased cell death and vessel regression at embryonic day 10.5 (E10.5). Deletion of TNF signaling largely rescued endothelial cell death in TAK1-deficient embryos at E10.5. However, embryos deficient in both TAK1 and TNF signaling still exhibited dilated capillary networks at E12.5. TAB2 deficiency caused reduced TAK1 activity, resulting in abnormal capillary blood vessels, similar to the compound deficiency of TAK1 and TNF signaling. Ablation of either TAK1 or TAB2 impaired cell migration and tube formation. Our results show that endothelial TAK1 signaling is important for 2 biologic processes in angiogenesis: inhibiting TNF-dependent endothelial cell death and promoting TNF-independent angiogenic cell migration.
Introduction
The first vascular plexus in embryos is formed by de novo aggregation of angioblasts (vasculogenesis). Thereafter, vessels are mainly formed by sprouting and splitting from preexisting vessels, processes that are referred to as sprouting angiogenesis and intussusceptive angiogenesis, respectively.1 Sprouting angiogenesis is promoted through a mechanism similar to axon guidance, in which sprout tip cells sense and migrate toward several guidance cues, including peptide growth factors such as VEGFs and semaphorins.2-5 Tip cells regulate the fate of neighboring endothelial cells through a Notch-mediated mechanism that promotes the formation of the sprout stalk and subsequently the formation of new branched vessels.1 Newly formed vessels are covered and stabilized by mural cells, including smooth muscle cells and pericytes.6,7 Functional interactions between endothelial cells and mural cells through several growth factor signaling pathways, including the PDGF, TGF-β, and angiopoietin family pathways, are essential for cell differentiation, pericyte recruitment, and vessel integrity.2,8 In addition to endothelial and mural cells, macrophages play a critical role in promoting vascular anastomosis.9,10 These angiogenic processes are modulated by environmental factors including hypoxia,11 inflammatory cytokines,12 and blood flow.13 However, the mechanisms by which embryonic angiogenesis is regulated remain elusive.
TGF-β activated kinase 1 (TAK1) is a member of the MAPK kinase kinase (MAPKKK) family and is activated by various stimuli including TLR ligands, TGF-β, and proinflammatory cytokines such as IL-1 and TNF.14 TAK1 is an indispensable signaling molecule in innate immune signaling through activation of NF-κB, AP-1, and other molecules.14-16 Recent studies using gene-targeted mice have revealed a role for TAK1 signaling in in vivo tissue homeostasis. We have reported previously that TAK1 is important for preventing TNF-induced epithelial cell death in the epidermis and the intestinal epithelium.17-20 T cell–specific knockout of Tak1 results in impaired T-cell survival and differentiation.21,22 The TAK1 protein is expressed ubiquitously during embryogenesis until around embryonic day 10.5 (E10.5),23 and germline deficiency of the Tak1 gene causes embryonic lethality at E9.5-10.5, which is associated with multitissue defects including vascular abnormalities.15,24 These TAK1-deficient embryos exhibit dilated blood vessels associated with the loss of smooth muscle differentiation. The present study investigated the role of endothelial-derived TAK1 signaling in embryonic vascular formation.
TAK1 has 2 important binding partners, TAK1 binding protein 1 (TAB1) and TAK1 binding protein 2 (TAB2).25 TAB1 and TAB2 are structurally unrelated and bind to 2 distinct regions in TAK1, the kinase domain and the C-terminal coiled-coil domain, respectively. Both TAB1 and TAB2 function to activate TAK1 but via different signaling pathways. TAB1 is essential for osmotic stress-induced TAK1 activation, but it is not required for IL-1– or TNF-induced TAK1 activation.15,26 TAB2 and the closely related, functionally redundant TAB3 protein mediate TNF- and IL-1–induced TAK1 activation.25 Disruption of the Tab1 gene causes embryonic lethality at E15.5-18.5 with developmental defects including failures of heart and lung morphogenesis.27,28 Germline deletion of the Tab2 gene is reported to cause liver degeneration during embryogenesis and is lethal at E12.5.29 TAB2 has recently been implicated in heart development in humans30 and is known to be expressed in the endothelium.31 However, the role of TAB2 in embryonic angiogenesis has not yet been examined.
TAK1 plays a central role in numerous innate immune signaling pathways. Activators of TAK1 include TLR ligands and inflammatory cytokines, which have recently been shown to be major promoters of angiogenesis by modulating endothelial cell proliferation and cell migration in adult animals.32 Therefore, we hypothesized that TAK1 and its binding partners would play a role in modulating angiogenesis. To explore the potential roles of TAK1, TAB1, and TAB2 in angiogenesis, we used mice with endothelial cell–specific gene deletions of Tak1, Tab1, or Tab2. In the present study, we report that TAK1 and TAB2, but not TAB1, are involved in embryonic angiogenesis. Furthermore, we demonstrate that endothelial TAK1 and TAB2 signaling regulates 2 different biologic processes in angiogenesis, endothelial cell survival and migration, both of which are important for embryonic angiogenesis.
Methods
Mice
Tak1-floxed (Tak1flox/flox) mice,16 Tab1-floxed (Tab1flox/flox) mice,27 and Tab2-floxed (Tab2flox/flox) mice29 have been described previously and were backcrossed at least 5 times with C57BL/6 mice. Tnfr1-deficient (Tnfr1−/−) and Tie2-Cre mice in a C57/BL6 background and Rosa26-CreER mice in a mixed B6;129 background were obtained from The Jackson Laboratory.33-35 Because a 5-times backcross is not sufficient to rule out possible effects from background differences, all experiments were performed using littermate controls. Lung endothelial cells from Rosa26-CreER transgenic mice were isolated as described in “Isolation of lung endothelial cells,” and littermate controls were also used. Induction of Tak1 and Tab2 deletion in Rosa-CreER Tak1flox/flox and Rosa-CreER Tab2flox/flox mice was achieved by IP injection of tamoxifen (150 mg/kg body weight) for 3-4 consecutive days. Deletion of the Tak1, Tab1, Tab2, and Tnfr1 genes was confirmed by PCR. All animal experiments were conducted with the approval of the North Carolina State University Institutional Animal Care and Use Committee.
Cells
Human umbilical vein endothelial cells (HUVECs) were obtained from Invitrogen and cultured in M200PRF supplemented with LSGS (Invitrogen) and penicillin-streptomycin at 37°C in 5% CO2.
Whole-mount PECAM-1 staining
Embryos and yolk sacs were fixed in 4% paraformaldehyde in PBS at 4°C overnight, washed 3 times with PBS, dehydrated in methanol, bleached with 5% hydrogen peroxide in methanol for 5 hours at room temperature, and washed 3 times with 0.1% Tween 20 in PBS (PBST). Samples were then blocked with blocking buffer (0.1% BSA, 0.15% normal goat serum in PBST) for 2 hours at 4°C and incubated overnight with anti–PECAM-1 Ab (BD Biosciences; 1:500 dilution in blocking buffer) at 4°C. After 5 washes with PBST, samples were incubated with biotin- or Alexa Fluor 568–conjugated anti–rat Ab overnight and washed with PBST. Color was developed using a VECTASTAIN Elite ABC kit (Vector Laboratories). Bright-field and dark-field image captures of whole-mount embryo and yolk sac preparations were obtained using a stereomicroscope (SMZ1500; Nikon), a microscope (BX41; Olympus), a camera (CS30, Olympus), NIS-Elements F Version 3.0 software (Nikon), and CellSens Standard Version 1.5 software (Olympus).
Fluorescent staining and immunohistochemistry analysis
To detect cell death, the terminal deoxynucleotidyltransferase-mediated dUTP nick end-labeling (TUNEL) assay was performed on frozen sections using the DeadEnd TUNEL system (Promega) according to the manufacturer's instructions. To detect proliferating cells, mice were injected with 0.1 mg/g of bromodeoxyuridine 2 hours before sacrifice. Immunohistochemical analysis was performed using anti-bromodeoxyuridine Ab (BD Biosciences). Immunofluorescence staining was performed on frozen or formalin-fixed paraffin sections using PECAM-1 (BD Biosciences), α-smooth muscle actin (SMAα; Sigma-Aldrich), VWF (DKO), and collagen IV (Abcam) Abs. Bound Abs were visualized by Cy2-, Cy3-, Alexa Fluor 488–, or Alexa Fluor 568–conjugated secondary Abs (Chemicon). Nuclei were counterstained with DAPI. Fluorescent images were acquired on an upright microscope (BX41; Olympus) equipped with 20×/0.5, 40×/0.75, and 60×/1.25 UPlanFl objectives, a camera (ORCA ER, Hamamatsu Photonics or XM10, Olympus), and image-processing software (IPLab Version 3.9.4, SpectraService or Cellsens, Olympus). Individual images were cropped and assembled into figures using Adobe Photoshop Version CS5. Total lengths of vessels and branch points were determined in 3 randomly chosen areas (1 mm2 in total) of 4 independent yolk sacs.
Confocal imaging
Confocal images of yolk sacs were obtained with a confocal microscope (LSM 710; Carl Zeiss) using a 40 × /1.1 NA water immersion objective, and images were collected with Zen 2009 software (Carl Zeiss). The images were exported as full-resolution TIF files and processed in Photoshop CS5 to adjust brightness and contrast. Twelve- to 14-μm optical sections were used for Z-stack reconstitution.
Isolation of lung endothelial cells
Lungs were collected from 3 or more mice at the age of 6-8 weeks, washed in HBSS, and then minced into 1- to 2-mm pieces. The minced lungs were digested with 1 mg/mL of collagenase type 1 (Sigma-Aldrich) and 0.2 mg/mL of Dispase (Invitrogen) at 37°C for 1 hour with occasional agitation. The cellular digest was filtered through a 40-μm nylon mesh and washed several times with a medium supplemented with 10% bovine growth serum (Thermo). The mononuclear fraction was isolated using Percoll (GE Healthcare), and cells were incubated at 4°C for 1 hour with Dynabeads (Invitrogen) coated overnight with PECAM-1 Ab. Bead-bound endothelial cells were isolated magnetically and washed 4 times with DMEM and once with PBS. These purified endothelial cells were used for further study.
Immunoblotting
Yolk sacs were isolated from mice, washed with PBS, and homogenized with plastic pestles in complete lysis buffer (Active Motif). Lung endothelial cell extracts or HUVEC extracts were prepared using a standard cell lysis buffer described previously. Cell extracts were resolved on SDS-PAGE and transferred to Hybond-P membranes (GE Healthcare). The membranes were immunoblotted with various Abs and visualized with HRP-conjugated Abs against rabbit or mouse IgG using the ECL Western blotting system (GE Healthcare). TAK1, TAB1, and TAB2 Abs were described previously.14,25 Phospho-TAK1 (Thr-187), phospho-ERK (Thr-202/Tyr204), phospho-AKT (Ser-493), phospho-p38 (Thr-180/Tyr-182), ERK, and AKT Abs were obtained from Cell Signaling Technology; p38 and p65 Abs were from Santa Cruz Biotechnology; and β-actin Ab was from Sigma-Aldrich.
Transient siRNA
TAK1, TAB2, and nontargeting control siRNAs were from Sigma-Aldrich (TAK1 siRNA, 5′-GGCAAAGCAACAGAGUGAAUCUGGA-3′; Tab2 siRNA, 5′-CAUACAUGGUGUACCUCCACCUGUA-3′; and nontargeting siRNA, 5′-UUCUCCGAACGUGUCACGU-3′). HUVECs were trypsinized and collected. Cells were suspended in OPTI-MEM medium (Invitrogen) containing siRNA oligos and subjected to electroporation using a Gene Pulser Xcell (Bio-Rad).
Endothelial tube formation assay
Twenty-four-well plates were coated with 100 μL of Geltrex (Invitrogen) at 37°C for 30 minutes. HUVECs were seeded on Geltrex and cultured at 37°C in 5% CO2. Tube formation was visualized by the addition of Calcein AM (Invitrogen) and observed by fluorescence microscopy (Olympus CRX41). For some samples, 5Z-7-oxozeaenol was added at the time of cell seeding on Geltrex.
Endothelial migration assay
HUVEC migration was assayed using COSTAR Transwells. The Transwells were coated with collagen type 1 at 5 μg/cm2 (BD Biosciences). 2 × 105 HUVECs were suspended in 200 μL of M200PRF medium and seeded into the upper chamber. The lower chamber contained M200PRF medium with or without 8% serum. Cells were allowed to migrate across an 8.0-μm pore size membrane at 37°C in 5% CO2 for 6 hours and stained with DAPI. The numbers of migrating cells in the total cell population counted were determined.
Flow cytometric analysis
Embryos and yolk sacs were digested with collagenase type 1 and dispase at 37°C for 30 minutes. The cellular digests from lungs or yolk sacs were filtered through a 40-μm nylon mesh and washed 3 times with DMEM. Cells were incubated with PE-Ter119 (BioLegend) or FITC-CD71 and APC-F4/80 (eBiosciences) at 4°C for 45 minutes. For cell death analysis, HUVEC cells were incubated with 7-amino-actinomycin D (Invitrogen) for 30 minutes. Fluorescence was analyzed with an FACS LSR II flow cytometer (BD Biosciences) and FlowJo Version 8.8.7 software.
Results
Ablation of TAK1 in endothelial cells impairs embryonic angiogenesis and causes lethality
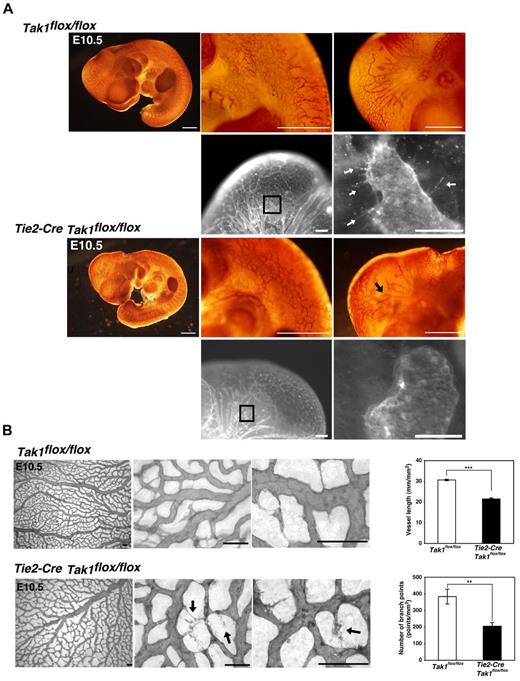
TAK1 is expressed ubiquitously in embryos23 and in the present study, we confirmed that Tak1 mRNA is expressed in the endothelium at E10.5 (supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article). To investigate the role of TAK1 in endothelial cells, we used Tie2-Cre/loxP–based conditional mutagenesis.33 We crossed Tie2-Cre Tak1flox/+ mice with Tak1flox/flox mice and analyzed the embryos, which had 4 different genotypes: Tak1flox/flox, Tak1flox/+ (control), Tie2-Cre Tak1flox/+ (endothelial-specific hetero-knockout), and Tie2-Cre Tak1flox/flox (endothelial-specific TAK1 knockout, TAK1ecko). The endothelial-specific deletion of Tak1 was confirmed by in situ hybridization (supplemental Figure 1A). Whereas the control and hetero-knockout littermates did not exhibit any overt abnormalities, TAK1ecko embryos were found to be lethal between E10.5 and E11.5 (supplemental Table 1A). To examine whether this embryonically lethal phenotype is associated with vascular abnormalities, we analyzed whole-mount tissue samples by immunohistochemical staining with the endothelial cell marker PECAM-1 (Figure 1A-B). Although the growth of TAK1ecko embryos was not markedly impaired, they exhibited defects in vascular development at E10.5 compared with control littermates. TAK1ecko embryos appeared to initiate plexus development, but the vasculature was disorganized and characterized by truncated vessels (Figure 1A). Vessel sprouting was found to be impaired in TAK1ecko head vessels (Figure 1A black and white images). All of the TAK1ecko embryos analyzed from 6 different litters (n = 10) exhibited similar abnormalities. We also examined vessel structure in the control and TAK1ecko yolk sacs (Figure 1B and supplemental Figure 2). Whereas the wild-type yolk sac was covered by highly branched blood vessels, the TAK1ecko yolk sac developed vessel networks with fewer branches, (Figure 1B), and the abnormalities were seen in all the areas of the yolk sac (supplemental Figure 2). We found that in the TAK1ecko yolk sac, vessels often exhibited unusual deterioration (Figure 1B arrows in higher magnifications). Quantitation of the lengths of vessels and the number of branches showed that these were significantly decreased in the TAK1ecko yolk sac compared with wild-type (Figure 1B). In contrast to capillary vessels, aortas were structurally indistinguishable between all of the wild-type and TAK1ecko embryos analyzed (n = 4; supplemental Figure 3A). Vascular smooth muscle cells and pericytes visualized by SMAα staining appeared to be recruited normally to TAK1ecko aortas and capillaries in the yolk sac (supplemental Figure 3A-B).
Endothelial-specific TAK1 deficiency impairs vascular development. (A) Whole-mount colorimetric PECAM-1 staining of control (Tak1flox/flox) and TAK1ecko (Tie2-Cre Tak1flox/flox) embryos at E10.5 (color images). Pictures are representative of 6 independent litters (n = 10). Black arrow indicates truncated vessel. Whole-mount fluorescent PECAM-1 staining of control (Tak1flox/flox) and TAK1ecko (Tie2-Cre Tak1flox/flox) are also shown (black and white images). Areas indicated in lower-magnification images are shown at a higher magnification. Arrows indicate sprouting vessels. Scale bar indicates 200 μm. (B) Whole-mount PECAM-1 staining of control (Tak1flox/flox) and TAK1ecko (Tie2-Cre Tak1flox/flox) yolk sacs at E10.5. Vessel lengths and branch points were determined in 3 randomly chosen areas from 3 different yolk sacs (graphs). Data are shown as means ± SD (n = 3). ***P < .001; **P < .01.
Endothelial-specific TAK1 deficiency impairs vascular development. (A) Whole-mount colorimetric PECAM-1 staining of control (Tak1flox/flox) and TAK1ecko (Tie2-Cre Tak1flox/flox) embryos at E10.5 (color images). Pictures are representative of 6 independent litters (n = 10). Black arrow indicates truncated vessel. Whole-mount fluorescent PECAM-1 staining of control (Tak1flox/flox) and TAK1ecko (Tie2-Cre Tak1flox/flox) are also shown (black and white images). Areas indicated in lower-magnification images are shown at a higher magnification. Arrows indicate sprouting vessels. Scale bar indicates 200 μm. (B) Whole-mount PECAM-1 staining of control (Tak1flox/flox) and TAK1ecko (Tie2-Cre Tak1flox/flox) yolk sacs at E10.5. Vessel lengths and branch points were determined in 3 randomly chosen areas from 3 different yolk sacs (graphs). Data are shown as means ± SD (n = 3). ***P < .001; **P < .01.
Blood vasculature is known to be regulated not only by blood vessel–associated cells, but also by several other factors, including blood flow13 and macrophage-mediated sprout outgrowth and anastomosis.9,10 In the Tie2-Cre/loxP system, Cre-derived recombination also occurs in hematopoietic cells and heart endothelial cells, including endocardial cushion mesenchymal cells.33,36 Therefore, we examined whether defects in the blood cells and/or the heart could be involved in the capillary abnormalities observed in TAK1ecko embryos. At E10.5, TAK1ecko embryos exhibited both blood circulation (supplemental Figure 4) and heartbeat. F4/80+ (macrophages), CD71+Ter119+ (immature erythroid cells), and CD71-Ter119+ (mature erythroid cells, reticulocytes, and erythrocytes) were found to have developed normally in TAK1ecko embryos (supplemental Figure 5A-B). Heart morphogenesis in TAK1ecko embryos, including the development of cardiac cushion and trabeculation, was indistinguishable from the control littermates (supplemental Figure 6). We conclude that defects in the heart or macrophages were unlikely to be a major cause of the blood vessel abnormalities seen in TAK1ecko embryos, although the role of hematopoietic cell-derived TAK1 signaling in embryogenesis remains to be defined. Our results suggest that endothelial cell–derived TAK1 signaling is required for intact capillary formation.
TAK1 is essential for preventing TNF-induced cell death in embryonic endothelial cells
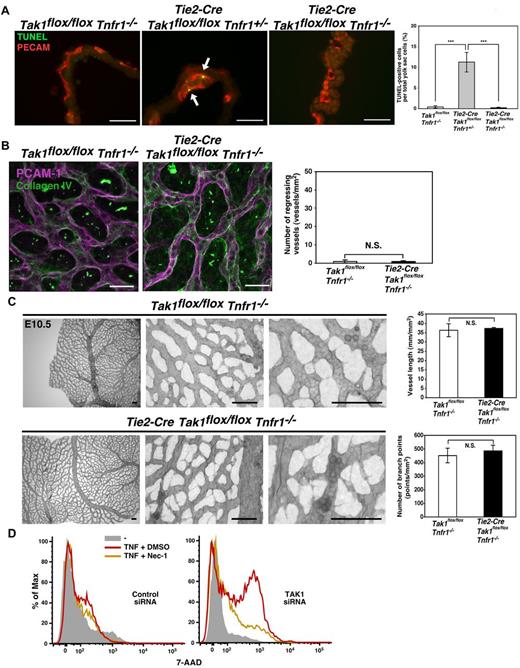
We next investigated the cause of impaired capillary vessels in TAK1ecko mice. Cell proliferation was not altered in the TAK1ecko yolk sacs (supplemental Figure 7A). Double staining for collagen IV and PECAM-1 revealed that several vessels in the TAK1ecko yolk sac were positive for collagen IV but negative for PECAM-1 (Figure 2A). These results indicate that vessels were formed but regressed in the presence of TAK1 deficiency. We have previously reported that ablation of TAK1 in the epidermis and the intestinal epithelium causes hypersensitivity to TNF-induced cell death.19,20 The additional deletion of TNF receptor 1 (Tnfr1) rescues epithelial cell death and restores tissue integrity.19,20 Therefore, in the present study, we examined whether the vessel defects observed in TAK1ecko embryos was associated with TNF-induced cell death. We observed a marked increase in TUNEL+ cells in the TAK1ecko yolk sac at E10.5, and these cells overlapped with PECAM-1+ cells (Figure 2B). To determine whether this increase in cell death was mediated through TNF signaling, we generated TAK1ecko mice in a TNFR1−/− background. Whereas TAK1ecko on a Tnfr1 heterozygous knockout, TNFR1+/−, exhibited endothelial cell death similar to TAK1ecko, its littermate double knockout, TAK1ecko TNFR1−/−, did not have any dead endothelial cells (Figure 3A). Collagen IV staining revealed that vessel regression was not present in TAK1ecko TNFR1−/− yolk sacs (Figure 3B). Vessel length and branching were also not impaired in TAK1ecko TNFR1−/− yolk sacs (Figure 3C and supplemental Figure 8A). The head and the trunk region of TAK1ecko TNFR1−/− embryos did not exhibit pronounced impairment in blood vessel structure (supplemental Figure 8B), and the embryos were viable at E10.5 (supplemental Table 1B). These results indicate that TAK1 is essential for preventing TNF-induced endothelial cell death, thereby allowing normal vessel formation.
Endothelial-specific TAK1 deficiency causes vessel regression and cell death at E10.5. (A) Confocal Z-stack images of whole-mount PECAM-1 and collagen IV staining of control (Tak1flox/flox) and TAK1ecko (Tie2-Cre Tak1flox/flox) yolk sacs at E10.5. Arrows indicate regions positive for collagen IV and negative for PECAM-1. Photos are representative of 3 independent litters (n = 3). Scale bar indicates 40 μm. The number of vessel regions that were positive for collagen IV but negative for PECAM-1 were defined as regressing vessels and were counted in 30 randomly chosen areas (total in 2 mm2) from 3 different yolk sacs (graph). Data are shown as means ± SD (n = 3). ***P < .001. (B) TUNEL (green) and PECAM-1 (red) staining of TAK1 control (Tak1flox/flox) and TAK1ecko (Tie2-Cre Tak1flox/flox) yolk sacs at E10.5. Arrows indicate TUNEL positive cells. Scale bar indicates 40 μm. The percentages of TUNEL+ cells in the total cell population of TAK1 control and TAK1ecko yolk sacs are shown. Data are shown as means ± SD (Tak1flox/flox, n = 5; Tie2-Cre Tak1flox/flox, n = 4). ***P < .001.
Endothelial-specific TAK1 deficiency causes vessel regression and cell death at E10.5. (A) Confocal Z-stack images of whole-mount PECAM-1 and collagen IV staining of control (Tak1flox/flox) and TAK1ecko (Tie2-Cre Tak1flox/flox) yolk sacs at E10.5. Arrows indicate regions positive for collagen IV and negative for PECAM-1. Photos are representative of 3 independent litters (n = 3). Scale bar indicates 40 μm. The number of vessel regions that were positive for collagen IV but negative for PECAM-1 were defined as regressing vessels and were counted in 30 randomly chosen areas (total in 2 mm2) from 3 different yolk sacs (graph). Data are shown as means ± SD (n = 3). ***P < .001. (B) TUNEL (green) and PECAM-1 (red) staining of TAK1 control (Tak1flox/flox) and TAK1ecko (Tie2-Cre Tak1flox/flox) yolk sacs at E10.5. Arrows indicate TUNEL positive cells. Scale bar indicates 40 μm. The percentages of TUNEL+ cells in the total cell population of TAK1 control and TAK1ecko yolk sacs are shown. Data are shown as means ± SD (Tak1flox/flox, n = 5; Tie2-Cre Tak1flox/flox, n = 4). ***P < .001.
TNFR1 deficiency rescues cell death and vessel regression in TAK1ecko. (A) TUNEL (green) and PECAM-1 (red) staining of control, Tak1flox/floxTnfr1−/−, TAK1ecko TNFR1 heterozygous knockout, Tie2-Cre Tak1flox/floxTnfr1+/− and double knockout, Tie2-Cre Tie2-Cre Tak1flox/flox Tnfr1−/−yolk sacs at E10.5. Arrows indicate TUNEL positive cells. Scale bar indicates 40 μm. The percentages of TUNEL+ cells in the total cell population of yolk sacs are shown. Data are shown as means ± SD (n = 3). ***P < .001. (B) Vessel regression was determined by collagen IV and PECAM-1 double staining at E10.5. The number of vessel regions that were positive for collagen IV but negative for PECAM-1 were defined as regressing vessels and were counted in 30 randomly chosen areas (total in 2 mm2) from 3 different yolk sacs (graph). Data are shown as means ± SD (n = 3). N.S. indicates not significant. (C) Whole-mount PECAM-1 staining of control TNFR1−/− (Tak1flox/floxTnfr1−/−) and TAK1ecko TNFR1−/− (Tie2-Cre Tak1flox/floxTnfr1−/−) yolk sacs at E10.5. Vessel lengths and branch points were determined in 3 randomly chosen areas from 3 different yolk sacs (graphs). Data are shown as means ± SD (n = 3). N.S. indicates not significant. (D) HUVECs were treated with control siRNA or siRNA targeting Tak1. The protein level of TAK1 is shown in supplemental Figure 9B. Cells were preincubated with vehicle (DMSO) or necrostatin-1 (20μM) for 1 hour and stimulated with 200 ng/mL of TNF for 6 hours. Cells were subjected to 7-amino-actinomycin D (7-AAD) analysis by FACS.
TNFR1 deficiency rescues cell death and vessel regression in TAK1ecko. (A) TUNEL (green) and PECAM-1 (red) staining of control, Tak1flox/floxTnfr1−/−, TAK1ecko TNFR1 heterozygous knockout, Tie2-Cre Tak1flox/floxTnfr1+/− and double knockout, Tie2-Cre Tie2-Cre Tak1flox/flox Tnfr1−/−yolk sacs at E10.5. Arrows indicate TUNEL positive cells. Scale bar indicates 40 μm. The percentages of TUNEL+ cells in the total cell population of yolk sacs are shown. Data are shown as means ± SD (n = 3). ***P < .001. (B) Vessel regression was determined by collagen IV and PECAM-1 double staining at E10.5. The number of vessel regions that were positive for collagen IV but negative for PECAM-1 were defined as regressing vessels and were counted in 30 randomly chosen areas (total in 2 mm2) from 3 different yolk sacs (graph). Data are shown as means ± SD (n = 3). N.S. indicates not significant. (C) Whole-mount PECAM-1 staining of control TNFR1−/− (Tak1flox/floxTnfr1−/−) and TAK1ecko TNFR1−/− (Tie2-Cre Tak1flox/floxTnfr1−/−) yolk sacs at E10.5. Vessel lengths and branch points were determined in 3 randomly chosen areas from 3 different yolk sacs (graphs). Data are shown as means ± SD (n = 3). N.S. indicates not significant. (D) HUVECs were treated with control siRNA or siRNA targeting Tak1. The protein level of TAK1 is shown in supplemental Figure 9B. Cells were preincubated with vehicle (DMSO) or necrostatin-1 (20μM) for 1 hour and stimulated with 200 ng/mL of TNF for 6 hours. Cells were subjected to 7-amino-actinomycin D (7-AAD) analysis by FACS.
We also investigated the mechanisms by which TNF kills TAK1-deficient endothelial cells. TNF induces cell death through 2 different mechanisms: caspase-dependent apoptosis, which is prevented by the NF-κB and MAPK pathways, and receptor interacting protein (RIP1) kinase–dependent necrosis.37 Although TAK1 is an activator of NF-κB and MAPKs such as p38 in other cell types, including primary brain endothelial cells,38 ablation of TAK1 did not alter the levels of NF-κB and p38 activity in the yolk sac at E10.5 (supplemental Figure 9A). Therefore, NF-κB and p38 are unlikely to be involved in prevention of TNF-induced cell death. To further understand the mechanisms by which TAK1 regulates angiogenesis, HUVECs were treated with siRNAs targeting TAK1 (supplemental Figure 9B). TAK1-knockdown HUVECs did not exhibit any noticeable differences in cell viability, but were sensitive to TNF-induced cell death (Figure 3D and supplemental Figure 9C), which is consistent with the in vivo phenotypes of TAK1ecko. We found that an inhibitor of RIP1 kinase, necrostatin-1, could block TNF-induced cell death in TAK1-knockdown HUVECs (Figure 3D and supplemental Figure 9C), suggesting that TAK1 prevents TNF-induced RIP1-dependent cell death in endothelial cells.
TAK1 and TAB2 are important for embryonic angiogenesis via modulation of a biologic process independent of the prevention of TNF-induced cell death
Although deletion of Tnfr1 rescued vessel formation in TAK1ecko embryos at E10.5 (Figure 3), these TAK1ecko TNFR1−/− embryos still died at approximately E11.5-E12.5 (Figure 4A and supplemental Table 1B) and exhibited impaired blood vessel development in embryos at E11.5-E12.5 (Figure 4B-C). Interestingly, the yolk sac blood vessels exhibited very different abnormalities from those seen in TAK1ecko (Figure 1B). The TAK1ecko TNFR1−/− yolk sac capillaries were dilated and exhibited an almost lagoon-like structure (Figure 4C). The dilated vessels were covered by collagen IV (supplemental Figure 10), indicating no vessel regression. The number of branches and sprouting was significantly decreased in TAK1ecko TNFR1−/− yolk sacs (Figure 4C bottom graphs), whereas cell proliferation was not altered (supplemental Figure 7B). These results show that whereas the endothelial cell death observed in TAK1ecko embryos was due to TNF signaling, TAK1 has other roles in embryonic angiogenesis. The TAK1ecko TNFR1−/− abnormalities resemble those previously reported for neuropilin and angiomotin-knockout embryos, which also show a lagoon-like vessel structure.39,40 Neuropilin and angiomotin are associated with endothelial angiogenic activities, including cell migration. Therefore, TAK1 regulates embryonic angiogenesis by a mechanism independent of cell death but related to other processes such as endothelial cell migration.
TAK1 is important for vascular development independent of suppression of TNF-induced cell death. (A) Percentages of viable TAK1ecko, TAB1ecko, and TAB2ecko embryos in a wild-type or Tnfr1−/− background are shown. PN indicates postnatal. (B-C) Whole-mount PECAM-1 staining of control TNFR1−/− (Tak1flox/floxTnfr1−/−) and TAK1ecko TNFR1−/− (Tie2-Cre Tak1flox/floxTnfr1−/−) embryos at E11.5 (B) and yolk sacs at E12.5 (C). Photos show representative specimens from 5 embryos. Vessel lengths, branch points, and the number of sprouts were determined in 3 randomly chosen areas from 3 different yolk sacs (graphs). Sprouts were defined as endothelial protrusions from the vascular loops that did not branch or fuse with other vessels. Data are shown as means ± SD (n = 3). Scale bar indicates 200 μm in embryos, 80 μm in yolk sacs. **P < .01; *P < .05.
TAK1 is important for vascular development independent of suppression of TNF-induced cell death. (A) Percentages of viable TAK1ecko, TAB1ecko, and TAB2ecko embryos in a wild-type or Tnfr1−/− background are shown. PN indicates postnatal. (B-C) Whole-mount PECAM-1 staining of control TNFR1−/− (Tak1flox/floxTnfr1−/−) and TAK1ecko TNFR1−/− (Tie2-Cre Tak1flox/floxTnfr1−/−) embryos at E11.5 (B) and yolk sacs at E12.5 (C). Photos show representative specimens from 5 embryos. Vessel lengths, branch points, and the number of sprouts were determined in 3 randomly chosen areas from 3 different yolk sacs (graphs). Sprouts were defined as endothelial protrusions from the vascular loops that did not branch or fuse with other vessels. Data are shown as means ± SD (n = 3). Scale bar indicates 200 μm in embryos, 80 μm in yolk sacs. **P < .01; *P < .05.
To further investigate the mechanisms by which TAK1 regulates angiogenesis, we examined the roles of 2 TAK1 binding proteins, TAB1 and TAB2, which are known to activate TAK1 in response to stress and inflammatory cytokines, respectively.25,26 Because the roles of TAB1 and TAB2 in TAK1 signaling during embryogenesis have not yet been determined, we undertook an analysis of mice harboring endothelial cell–specific Tab1 (TAB1ecko) or Tab2 (TAB2ecko) deletions. TAB1ecko mice were born at a normal Mendelian ratio and did not exhibit any gross abnormalities (Figure 4A and supplemental Table 2A). We also did not observe any overt abnormalities in the blood vessel structure in TAB1ecko embryos (supplemental Figure 11). TAB2 is known to be expressed in the endothelium.30 We confirmed that Tab2 mRNA was expressed and specifically deleted in the endothelium of TAB2ecko embryos and yolk sacs at E12.5 (supplemental Figure 1B). TAB2ecko embryos were lethal at approximately E12.5 and exhibited blood vessel abnormalities very similar to those seen in TAK1ecko TNFR1−/− embryos (Figure 4A, Figure 5A-B, and supplemental Table 2B). All of the TAB2ecko embryos analyzed (n = 4) exhibited lagoon-like and less-branched vessel structures in the yolk sac (Figure 5B). TAB2ecko embryos did not show any pronounced increase in TUNEL+ endothelial cells (supplemental Figure 12A), and cell proliferation was not altered (supplemental Figure 12B). TNFR1 deficiency did not rescue either defective vessel formation or lethality in TAB2ecko embryos (Figure 4A, supplemental Figure 12C, and supplemental Table 2C). These results suggest that TAB2 is important for the TNF-independent TAK1 signaling regulating embryonic angiogenesis.
TAB2 is important for TNF-independent TAK1 regulation of embryonic angiogenesis. (A-B) Whole-mount PECAM-1 staining of TAB2 control (Tab2flox/flox) and TAB2ecko (Tie2-Cre Tab2flox/flox) embryos and yolk sacs at E12.5. Photos show representative specimens from 4 embryos. Arrows indicate truncated vessels. Vessel lengths and branch points were determined in 3 randomly chosen areas from 3 different yolk sacs (graphs). Data are shown as means ± SD (n = 3). Scale bar indicates 200 μm in embryos, 80 μm in yolk sacs. ***P < .001; *P < .05.
TAB2 is important for TNF-independent TAK1 regulation of embryonic angiogenesis. (A-B) Whole-mount PECAM-1 staining of TAB2 control (Tab2flox/flox) and TAB2ecko (Tie2-Cre Tab2flox/flox) embryos and yolk sacs at E12.5. Photos show representative specimens from 4 embryos. Arrows indicate truncated vessels. Vessel lengths and branch points were determined in 3 randomly chosen areas from 3 different yolk sacs (graphs). Data are shown as means ± SD (n = 3). Scale bar indicates 200 μm in embryos, 80 μm in yolk sacs. ***P < .001; *P < .05.
TAB2 activates TAK1 in endothelial cells
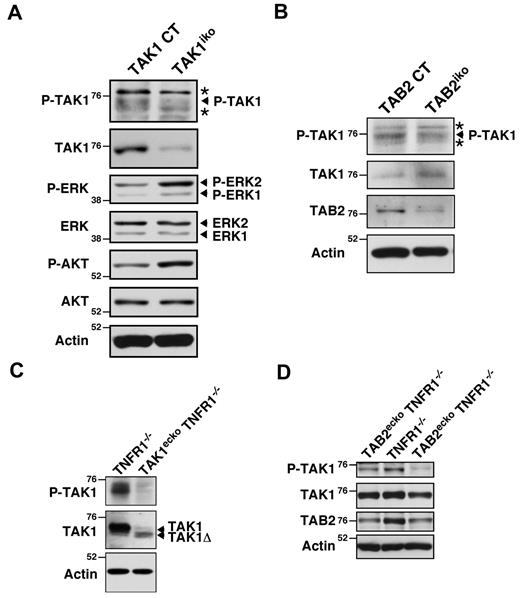
To investigate whether TAB2 activates TAK1 in endothelial cells, we examined TAK1 activity in lung endothelial cells and yolk sacs in vivo. We generated mice in which deletion of Tak1 or Tab2 could be induced in a non-tissue-specific manner (Rosa26-CreER Tak1flox/flox, TAK1iko and Rosa26-CreER Tab2flox/flox, TAB2iko, respectively). In this system, the Tak1 or Tab2 genes are deleted in various tissues, including the endothelium, on tamoxifen treatment. Lung endothelial cells were isolated from 6- to 8-week-old TAK1iko mice after tamoxifen treatment using magnetic beads coated with PECAM-1 Ab. TAK1 activity was determined by immunoblotting for the active form of TAK1, which is phosphorylated at Thr-187 within the activation loop of the kinase.41 We confirmed that endothelial cell fractions from the TAK1iko lung after tamoxifen treatment had reduced TAK1 activity (Figure 6A). We next analyzed TAK1 activity in TAB2-deficient endothelial cells and found that TAK1 activity was also reduced in TAB2iko lung endothelial cells (Figure 6B). The amounts of activated ERK and AKT, which are downstream targets of VEGF signaling, were not lower, but rather were higher in TAK1-deficient lung endothelial cells compared with wild-type (Figure 6A). This might suggest that TAK1 signaling is not involved in VEGF signaling, which is discussed further later in the text.
TAB2 activates TAK1 in the endothelium. (A) TAK1 control (Tak1flox/flox; TAK1 CT) and Rosa26-Cre Tak1flox/flox (TAK1iko) mice were treated with tamoxifen and lung endothelial cells were purified by magnetic beads coated with PECAM-1 Ab. The purified endothelial cells were analyzed by immunoblotting. (B) Lung endothelial cells from TAB2 control (Tab2flox/flox; TAB2 CT) and Rosa26-Cre Tab2flox/flox (TAB2iko) mice were analyzed by immunoblotting. (C) Cell lysates from control TNFR1−/− (Tak1flox/flox Tnfr1−/−) and TAK1ecko TNFR1−/− (Tie2-Cre Tak1flox/flox Tnfr1−/−) yolk sacs at E10.5 were analyzed by immunoblotting. (D) Cell lysates from TNFR1−/− (Tab2flox/flox Tnfr1−/−) and TAB2ecko TNFR1−/− (Tie2-Cre Tab2flox/flox Tnfr1−/−) yolk sacs at E11.5 and E12.5, respectively, were analyzed by immunoblotting. One control and 2 TAB2ecko TNFR1−/− embryos from the same litter were analyzed. Molecular weight markers are shown (kDa). Asterisks indicate nonspecific bands.
TAB2 activates TAK1 in the endothelium. (A) TAK1 control (Tak1flox/flox; TAK1 CT) and Rosa26-Cre Tak1flox/flox (TAK1iko) mice were treated with tamoxifen and lung endothelial cells were purified by magnetic beads coated with PECAM-1 Ab. The purified endothelial cells were analyzed by immunoblotting. (B) Lung endothelial cells from TAB2 control (Tab2flox/flox; TAB2 CT) and Rosa26-Cre Tab2flox/flox (TAB2iko) mice were analyzed by immunoblotting. (C) Cell lysates from control TNFR1−/− (Tak1flox/flox Tnfr1−/−) and TAK1ecko TNFR1−/− (Tie2-Cre Tak1flox/flox Tnfr1−/−) yolk sacs at E10.5 were analyzed by immunoblotting. (D) Cell lysates from TNFR1−/− (Tab2flox/flox Tnfr1−/−) and TAB2ecko TNFR1−/− (Tie2-Cre Tab2flox/flox Tnfr1−/−) yolk sacs at E11.5 and E12.5, respectively, were analyzed by immunoblotting. One control and 2 TAB2ecko TNFR1−/− embryos from the same litter were analyzed. Molecular weight markers are shown (kDa). Asterisks indicate nonspecific bands.
To further examine the role of TAB2 in modulating TAK1 activity during embryogenesis, we measured TAK1 activity in yolk sac extracts from TAK1ecko TNFR1−/− and TAB2ecko TNFR1−/− mice (Figure 6C-D). Cre-mediated recombination in Tak1flox/flox mice results in the expression of a truncated, kinase-negative form of TAK1 (TAK1Δ) that is usually less abundant compared with the full-length of TAK1, presumably because of decreased protein stability.16 We detected a small amount of TAK1Δ in TAK1ecko TNFR1−/− yolk sacs and a diminished amount of active TAK1 (Figure 6C). The results from one control (TNFR1−/−) and 2 TAB2ecko TNFR1−/− littermate embryos are shown in Figure 6D. The abundance of TAB2 was decreased and TAK1 activity was reduced in TAB2ecko TNFR1−/− yolk sacs compared with control littermates. These results suggest that TAB2 activates TAK1 in the yolk sac endothelium. However, there remained detectable levels of active TAK1 in TAB2ecko TNFR1−/− yolk sacs. We assume that some of this activated TAK1 protein was from nonendothelial cells in the yolk sac. An alternative but not exclusive possibility is a partial compensation of the loss of TAB2 by a structurally related TAB3.42 TAB2 might partially mediate TAK1 activation in embryonic endothelial cells, and the partially reduced TAK1 activity in TAB2-deficient cells may be enough to prevent TNF-induced cell death but not enough to promote other TAK1-dependent angiogenic processes.
Ablation of TAK1 and TAB2 impairs in vitro angiogenic activity and cell migration
The results of the present study indicate that TAK1 is required for preventing TNF-induced endothelial cell death, and that TAK1 and TAB2 are critically involved in angiogenic processes other than preventing cell death. We investigated the mechanism of TNF-independent abnormality in the TAK1- and TAB2-deficient endothelium. Because TAK1- and TAB2-knockdown HUVECs did not exhibit any increased cell death in the absence of exogenous TNF (Figure 3D and supplemental Figure 9C), the following experiments were designed to determine the cell death–independent role of the TAK1-TAB2 pathway. We first investigated whether TAK1 signaling is involved in the VEGF signaling pathway, one of the major pathways regulating angiogenesis.4 We examined VEGF-induced activation of ERK in control and TAK1-knockdown HUVECs, but found no obvious differences (supplemental Figure 13A). This is consistent with the observation that activation of ERK and AKT, which also function downstream of VEGF, was not decreased in TAK1-deficient endothelial cells in vivo (Figure 6A). We also noted that the levels of VEGF-A in yolk sacs were not altered by TAK1 deficiency (supplemental Figure 13B). Therefore, TAK1 appears to be unimportant for VEGF signaling, but rather is involved in some VEGF-independent aspects of angiogenesis.
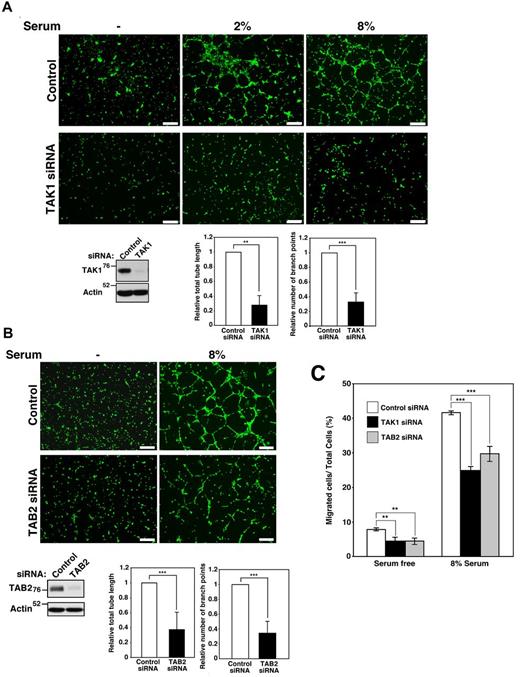
To determine whether endothelial TAK1 and TAB2 signaling is involved in angiogenic processes, TAK1- and TAB2-knockdown HUVECs were examined in an in vitro tube-formation assay. Increasing serum concentrations enhanced tube formation in control HUVECs, whereas both tube lengths and branch numbers were diminished in TAK1-knockdown HUVECs (Figure 7A). 5Z-7-oxozeaenol, an inhibitor of TAK1 kinase activity,43 inhibited tube formation (supplemental Figure 14). This indicates that TAK1 kinase activity is required for angiogenic activity. Furthermore, TAB2-knockdown HUVECs exhibited reduced tube formation, similar to TAK1-knockdown cells (Figure 7B). Therefore, TAK1 and TAB2 are essential for in vitro tube formation in endothelial cells. Because endothelial cell migration is essential for tube formation and angiogenesis, we next examined the cell migration of TAK1- and TAB2-knockdown HUVECs using Transwell chambers. TAK1- and TAB2-knockdown HUVECs exhibited less cell migration both in basal random and serum-stimulated migrations compared with control HUVECs (Figure 7C). Therefore, TAK1 and TAB2 are important for endothelial cell migration. In summary, our results show that deletion of the Tak1 gene causes abnormal angiogenesis through 2 mechanisms: TNF-induced endothelial cell death and impaired cell migration. Deletion of the Tab2 gene affects angiogenesis by reducing TAK1 activity, resulting in impaired cell migration (supplemental Figure 15). Both endothelial cell survival and TAK1-dependent cell migration are essential for angiogenesis in embryos.
TAK1 signaling is important for tube formation and endothelial cell migration. (A) HUVECs were treated with control siRNA or siRNA targeting Tak1. Tube formation was observed with increasing concentrations of serum after 10 hours. Scale bar indicates 50 μm. Cells were visualized by Calcein AM, and total lengths of all formed tubes and branch points were measured. The lengths and branch points in TAK1-knockdown cells relative to those in controls are shown. Data are shown as means ± SD (n = 3). ***P < .001; **P < .01. The levels of TAK1 protein were analyzed by immunoblot. (B) HUVECs were treated with control siRNA or siRNA targeting Tab2. Tube formation was observed in response to serum after 10 hours. Scale bar indicates 50 μm. Total lengths of all formed tubes and branch points were measured, and the lengths and branch points in TAB2-knockdown cells relative to those in controls are shown. Data are shown as means ± SD (n = 5). ***P < .001 The levels of TAB2 protein were analyzed by immunoblotting. (C) The migration of TAK1- and TAB2-knockdown HUVECs was assayed using Transwells coated with collagen type 1. HUVECs were suspended in the medium and seeded in the upper chamber and the medium with or without 8% serum was added to the lower chamber. Cells were allowed to migrate across an 8.0-μm pore size membrane for 6 hours and stained with DAPI and the percentages of migrating cells in the total cell population were determined. Data are shown as means ± SD (n = 4). ***P < .001; **P < .01.
TAK1 signaling is important for tube formation and endothelial cell migration. (A) HUVECs were treated with control siRNA or siRNA targeting Tak1. Tube formation was observed with increasing concentrations of serum after 10 hours. Scale bar indicates 50 μm. Cells were visualized by Calcein AM, and total lengths of all formed tubes and branch points were measured. The lengths and branch points in TAK1-knockdown cells relative to those in controls are shown. Data are shown as means ± SD (n = 3). ***P < .001; **P < .01. The levels of TAK1 protein were analyzed by immunoblot. (B) HUVECs were treated with control siRNA or siRNA targeting Tab2. Tube formation was observed in response to serum after 10 hours. Scale bar indicates 50 μm. Total lengths of all formed tubes and branch points were measured, and the lengths and branch points in TAB2-knockdown cells relative to those in controls are shown. Data are shown as means ± SD (n = 5). ***P < .001 The levels of TAB2 protein were analyzed by immunoblotting. (C) The migration of TAK1- and TAB2-knockdown HUVECs was assayed using Transwells coated with collagen type 1. HUVECs were suspended in the medium and seeded in the upper chamber and the medium with or without 8% serum was added to the lower chamber. Cells were allowed to migrate across an 8.0-μm pore size membrane for 6 hours and stained with DAPI and the percentages of migrating cells in the total cell population were determined. Data are shown as means ± SD (n = 4). ***P < .001; **P < .01.
Discussion
The results of the present study demonstrate that TAK1 signaling drives 2 biologic processes involved in angiogenesis: inhibition of TNF-dependent endothelial cell death and promotion of vessel formation resulting from increased cell migration (supplemental Figure 15). Endothelial cell proliferation and survival are regulated by a complex signaling network, and VEGF is known to play major roles, including activation of antiapoptotic protein Bcl-w.44 However, our results suggest that TAK1 signaling modulates endothelial cell survival through a VEGF-independent manner and that TAK1 prevents TNF-induced cell death by modulating RIP1-dependent necrosis. TAK1 is an indispensable intermediate of TNF and IL-1 signaling pathways leading to NF-κB and MAPKs, which are known to be important for prevention of TNF-induced apoptosis by up-regulating inhibitors of caspases.37 Recently, TAK1 has been reported to be essential for MAPK p38 activation in brain endothelial cells.38 Therefore, we initially anticipated that TAK1 deficiency would cause endothelial cell death because of loss of NF-κB and/or MAPK activity. However, the activity of NF-κB and MAPKs was not altered, at least not in TAK1-deficient yolk sacs at E10.5, indicating that these are regulated by signaling pathways other than TAK1-mediated ones. Several earlier studies report that ablation of NF-κB in the endothelium does not cause embryonic vascular abnormality,45,46 which is consistent with the idea that TAK1 signaling regulates vessel formation through a NF-κB–independent mechanism. We found that TAK1-deficient endothelial cell death was effectively rescued by inhibition of RIP1 kinase, suggesting that TNF-induced necrosis, also called necroptosis, is involved in this cell death. TAK1 is likely to modulate the RIP1-RIP3 pathway in endothelial cells, which warrants further research.
Deletion of TNFR1 rescued endothelial cell death but not vessel defects at E12.5 in TAKecko or TAB2ecko embryos. We showed that TAK1-deficient endothelial cells did not die in the absence of TNF signaling. Therefore, the vessel defects in TAK1ecko TNFR1−/− and TAB2ecko TNFR1−/− embryos are not because of cell death. Our results indicate that TAK1 and TAB2 are also important for endothelial cell migration. The RIP1-RIP3 pathway has recently been reported to regulate mitochondrial function and may affect energy metabolism.38 Modulation of the RIP1-RIP3 pathway by TAK1 signaling may also be involved in endothelial cell function. Endothelial cell proliferation, migration, and survival are known to be regulated through a complex signaling network. The results of the present study demonstrate that TAK1 signaling is also required for these processes in vivo.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr E. Johannes for confocal microscopy imaging; Dr S. Akira for Tak1-floxed and Tab2-floxed mice; and S. Elliott, L. Hester, and J. Dow for technical support.
This work was supported by the National Institutes of Health (grants GM068812 and GM084406 to J.N.-T.). S.M. was supported by Young Research Fellowship, Japan Society for the Promotion of Science.
National Institutes of Health
Authorship
Contribution: S.M. and M.I. conducted the experiments; S.M., Y.K., Y.M., K.M., and J.N.-T. designed the study and analyzed the results; and S.M. and J.N-T wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
The current affiliation for M.I. is Research Institute for Radiation Biology and Medicine, Hiroshima University, Hiroshima, Japan.
Correspondence: Jun Ninomiya-Tsuji, Department of Environmental and Molecular Toxicology, North Carolina State University, Campus Box 7633, Raleigh, NC 27695-7633; e-mail: Jun_Tsuji@ncsu.edu.




This feature is available to Subscribers Only
Sign In or Create an Account Close Modal