Abstract
Rozrolimupab, a recombinant mixture of 25 fully human RhD-specific monoclonal antibodies, represents a new class of recombinant human antibody mixtures. In a phase 1 or 2 dose escalation study, RhD+ patients (61 subjects) with primary immune thrombocytopenia received a single intravenous dose of rozrolimupab ranging from 75 to 300 μg/kg. The primary outcome was the occurrence of adverse events. The principal secondary outcome was the effect on platelet levels 7 days after the treatment. The most common adverse events were headache and pyrexia, mostly mild, and reported in 20% and 13% of the patients, respectively, without dose relationship. Rozrolimupab caused an expected transient reduction of hemoglobin concentration in the majority of the patients. At the dose of 300 μg/kg platelet responses, defined as platelet count ≥ 30 × 109/L and an increase in platelet count by > 20 × 109/L from baseline were observed after 72 hours and persisted for at least 7 days in 8 of 13 patients (62%). Platelet responses were observed within 24 hours in 23% of patients and lasted for a median of 14 days. Rozrolimupab was well tolerated and elicited rapid platelet responses in patients with immune thrombocytopenia and may be a useful alternative to plasma-derived products. This trial is registered at www.clinicaltrials.gov as #NCT00718692.
Introduction
Primary immune thrombocytopenia (ITP) is an autoimmune disease mediated by antiplatelet antibodies that cause opsonization of platelets and elimination through binding to FcγR-bearing phagocytic cells and subsequent phagocytosis.1 In addition, the same autoantibodies bind to megakaryocytes and impair platelet production.1
The annual incidence of ITP in the United States is estimated to be ∼ 16 000 cases.2 Although the thrombocytopenia in ITP can be severe, most patients have only minor signs of bleeding. Persistently low platelet counts (< 20 × 109/L), however, are associated with an increased risk of serious bleeding, such as intracranial hemorrhage.3 Treatment is indicated for ITP associated with significant mucous membrane bleeding and is also indicated for patients with risk factors for bleeding (eg, hypertension, peptic ulcer disease, antiplatelet drug ingestion), in adult patients with a platelet count < 30 × 109/L, and in patients before surgical procedures.3
The initial treatment for ITP is composed of corticosteroids, intravenous high dose immunoglobulin (IVIg), or intravenous RhD immune globulin (anti-D). These compounds act primarily by interfering with platelet destruction4 and cytokine modulation.5 Immunomodulatory agents suppress the production of antiplatelet antibodies, but the use of immunosuppressive agents, corticosteroids, and later splenectomy may be associated with complications.
The platelet response to plasma-derived anti-D, including early onset and duration of response, seems to be dose dependent6,7 and at a dose of 75 μg/kg anti-D has an effect similar to IVIg.6,8 However, these data were derived from studies in which human plasma was the source of anti-D. Rozrolimupab, a mixture of 25 recombinant, fully human, RhD-specific monoclonal antibodies, represents the first in class of recombinant human monoclonal antibody mixtures9 that can be produced independently of human plasma supply. We here report safety and efficacy data from a trial of a single dose of rozrolimupab in nonsplenectomized adults with ITP.
Methods
Trial medication
Rozrolimupab is composed of 25 genetically unique IgG1 antibodies, all specific for the RhD erythrocyte antigen, derived from B-lymphocytes of 8 RhD-negative female donors with high serum antibody titers against RhD.9,10 The drug substance was produced by single batch manufacturing of a working cell bank composed of an equal mixture of 25 CHO cell lines, each expressing 1 particular human monoclonal antibody specific for RhD.9 Twenty antibodies have a κ-light chain and 5 antibodies a λ-light chain.10 The hypervariable regions of the heavy chains are homologous with published human anti-RhD sequences.10 Rozrolimupab is composed of antibodies specifically selected to recognize the complete D antigen expressed in > 99% of the human population, including rare D variants DIII, DIV, DVI, and DVII expressed in < 1% of the overall RhD-positive population.11 The rozrolimupab drug product exhibits stable composition in consecutive batches.12
Validated flow cytometry studies according to the European Pharmacopoeia demonstrated that rozrolimupab binds to RhD variants with a potency comparable with that of 2 marketed plasma-derived anti-D products, Rhophylac and WinRho.13 Explorative in vitro studies showed that rozrolimupab mediated specific phagocytosis and antibody-dependent cell-mediated cytotoxicity of RhD-expressing erythrocytes using either normal human mononuclear cells or THP-1 monocytic leukemia cells (ATCC) as effector cells. In the latter case, the potency of rozrolimupab appeared 5-fold reduced compared with plasma-derived anti-D products (data not shown).
Eligibility
Adult patients with ITP were enrolled at 27 investigational sites in Europe, Israel, and India. Eligibility criteria included the diagnosis of primary ITP14 documented response after initial therapy with corticosteroids, anti-D or IVIG defined as an increase in platelet count > 30 × 109/L, and a pretreatment platelet count of < 30 × 109/L. Key exclusion criteria included prior splenectomy, secondary thrombocytopenia, clinical splenomegaly, a history of abnormal bone marrow examination (except for megakaryocytosis), hemoglobin concentration < 2 g/dL below the lower limit of the normal range, and positive results of direct Coombs test.
Design
The trial was approved by independent ethics committees and the governmental authorities in each participating country, as required, and was carried out in accordance with the Declaration of Helsinki (October 1996). All patients provided oral and written informed consent before trial start.
This was an open-label, international, multicenter, exploratory dose finding, phase 1 or 2 trial. The trial was designed to include cohorts receiving a single dose of rozrolimupab 75, 100, 125, 150, 200, 250, 300, and 350 μg/kg, that each consisted of 5-11 patients. An Independent Data Monitoring Committee evaluated safety and efficacy between each dose escalation. Once the final dose level was achieved after this evaluation, the final dose cohort was repeated.
All patients received a single intravenous dose of rozrolimupab. For doses up to 150 μg/kg, rozrolimupab was given as a slow bolus intravenous injection of 3-5 minutes duration. For doses ≥ 150 μg/kg, rozrolimupab was added to a 100-mL sterile infusion bag containing 0.9% NaCl and infused over 15-20 minutes using a sterile inline filter (0.2 μm).
Patients were followed for 6 weeks.
The primary endpoint of the trial was the incidence and severity of adverse events (AEs), including serious adverse events (SAEs), during the 6-week trial period. Secondary safety endpoints included laboratory parameters (including maximum reduction of hemoglobin concentration), and presence of human anti–human antibodies.
Efficacy endpoints included measures of platelet response and use of rescue medication. Responders were defined as patients with platelet count ≥ 30 × 109/L and an increase in platelet count by > 20 × 109/L from baseline at 7 days after rozrolimupab treatment.
Analysis of hematology, clinical chemistry, coagulation, and hemolysis parameters was performed before treatment and at predefined intervals after treatment. The concentrations of IL-6, IL-10, monocyte chemotactic protein-1 (MCP-1), and TNF-α were measured by Luminex Technology kit (Merck Millipore).
Rozrolimupab serum concentrations were measured by flow cytometry as previously described.15 The lower limit of quantification was validated to 4 ng/mL in whole serum.
A double-antigen ELISA16 with detection limit of 6.3 ng/mL was used to detect human anti–human antibodies. Analysis for the FcγRIIA-131 H/R and FcγRIIIA-158 V/F genotypes was performed on DNA extracted from blood samples.17,18
No formal sample size calculations were performed. Linear regression was used to analyze possible relations between predictor and dependent variables.
Results
Characteristics of patients
Patients had a median age of 51 years and were predominantly female (64%; Table 1). The majority of patients (89%) were white.
Patient characteristics
| Rozrolimupab dose groups . | ||||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic . | 75 μg/kg . | 100 μg/kg . | 125 μg/kg . | 150 μg/kg . | 200 μg/kg . | 250 μg/kg . | 300 μg/kg . | All . |
| N | 11 | 10 | 10 | 5 | 6 | 6 | 13 | 61 |
| Sex | ||||||||
| Female | 3 (27.3%) | 9 (90.0%) | 6 (60.0%) | 4 (80.0%) | 5 (83.3%) | 5 (83.3%) | 7 (53.8%) | 39 (63.9%) |
| Male | 8 (72.7%) | 1 (10.0%) | 4 (40.0%) | 1 (20.0%) | 1 (16.7%) | 1 (16.7%) | 6 (46.2%) | 22 (36.1%) |
| Age, y | ||||||||
| Median | 51.0 | 40.0 | 41.0 | 52.0 | 60.5 | 42.5 | 55.0 | 51.0 |
| Range | 40.0-65.0 | 19.0-75.0 | 26.0-74.0 | 32.0-62.0 | 25.0-81.0 | 21.0-74.0 | 25.0-77.0 | 19.0-81.0 |
| Age category | ||||||||
| < 40 y | 0 | 4 (40.0%) | 5 (50.0%) | 2 (40.0%) | 2 (33.3%) | 3 (50.0%) | 4 (30.8%) | 20 (32.8%) |
| 40-60 y | 10 (90.9%) | 4 (40.0%) | 3 (30.0%) | 2 (40.0%) | 1 (16.7%) | 1 (16.7%) | 7 (53.8%) | 28 (45.9%) |
| > 60 y | 1 (9.1%) | 2 (20.0%) | 2 (20.0%) | 1 (20.0%) | 3 (50.0%) | 2 (33.3%) | 2 (15.4%) | 13 (21.3%) |
| Race | ||||||||
| Asian | 0 | 0 | 0 | 0 | 0 | 1 (16.7%) | 5 (38.5%) | 6 (9.8%) |
| White | 11 (100%) | 10 (100%) | 9 (90.0%) | 5 (100%) | 6 (100%) | 5 (83.3%) | 8 (61.5%) | 54 (88.5%) |
| Other | 0 | 0 | 1 (10.0%) | 0 | 0 | 0 | 0 | 1 (1.6%) |
| Body mass index, kg/m2 | ||||||||
| Median | 26.0 | 23.6 | 25.9 | 28.9 | 27.2 | 27.3 | 26.7 | 26.1 |
| Range | 18.5-36.8 | 18.0-30.8 | 21.0-38.1 | 22.7-30.5 | 18.7-31.3 | 20.1-38.7 | 21.1-40.7 | 18.0-40.7 |
| Time from first ITP diagnosis to screening, mo | ||||||||
| Median | 68 | 20 | 5 | 8 | 27 | 28 | 19 | 21 |
| Range | 1-166 | 4-354 | 2-187 | 4-141 | 1-117 | 3-223 | 1-273 | 1-354 |
| Baseline platelet count, × 109/L | ||||||||
| Median | 11.5 | 22.3 | 13.3 | 20.5 | 19.3 | 16.0 | 18.5 | 18.5 |
| Range | 5.0-24.5 | 10.5-26.5 | 3.0-26.5 | 4.5-21.5 | 3.0-26.0 | 6.0-26.0 | 7.3-27.0 | 3.0-27.0 |
| Rozrolimupab dose groups . | ||||||||
|---|---|---|---|---|---|---|---|---|
| Characteristic . | 75 μg/kg . | 100 μg/kg . | 125 μg/kg . | 150 μg/kg . | 200 μg/kg . | 250 μg/kg . | 300 μg/kg . | All . |
| N | 11 | 10 | 10 | 5 | 6 | 6 | 13 | 61 |
| Sex | ||||||||
| Female | 3 (27.3%) | 9 (90.0%) | 6 (60.0%) | 4 (80.0%) | 5 (83.3%) | 5 (83.3%) | 7 (53.8%) | 39 (63.9%) |
| Male | 8 (72.7%) | 1 (10.0%) | 4 (40.0%) | 1 (20.0%) | 1 (16.7%) | 1 (16.7%) | 6 (46.2%) | 22 (36.1%) |
| Age, y | ||||||||
| Median | 51.0 | 40.0 | 41.0 | 52.0 | 60.5 | 42.5 | 55.0 | 51.0 |
| Range | 40.0-65.0 | 19.0-75.0 | 26.0-74.0 | 32.0-62.0 | 25.0-81.0 | 21.0-74.0 | 25.0-77.0 | 19.0-81.0 |
| Age category | ||||||||
| < 40 y | 0 | 4 (40.0%) | 5 (50.0%) | 2 (40.0%) | 2 (33.3%) | 3 (50.0%) | 4 (30.8%) | 20 (32.8%) |
| 40-60 y | 10 (90.9%) | 4 (40.0%) | 3 (30.0%) | 2 (40.0%) | 1 (16.7%) | 1 (16.7%) | 7 (53.8%) | 28 (45.9%) |
| > 60 y | 1 (9.1%) | 2 (20.0%) | 2 (20.0%) | 1 (20.0%) | 3 (50.0%) | 2 (33.3%) | 2 (15.4%) | 13 (21.3%) |
| Race | ||||||||
| Asian | 0 | 0 | 0 | 0 | 0 | 1 (16.7%) | 5 (38.5%) | 6 (9.8%) |
| White | 11 (100%) | 10 (100%) | 9 (90.0%) | 5 (100%) | 6 (100%) | 5 (83.3%) | 8 (61.5%) | 54 (88.5%) |
| Other | 0 | 0 | 1 (10.0%) | 0 | 0 | 0 | 0 | 1 (1.6%) |
| Body mass index, kg/m2 | ||||||||
| Median | 26.0 | 23.6 | 25.9 | 28.9 | 27.2 | 27.3 | 26.7 | 26.1 |
| Range | 18.5-36.8 | 18.0-30.8 | 21.0-38.1 | 22.7-30.5 | 18.7-31.3 | 20.1-38.7 | 21.1-40.7 | 18.0-40.7 |
| Time from first ITP diagnosis to screening, mo | ||||||||
| Median | 68 | 20 | 5 | 8 | 27 | 28 | 19 | 21 |
| Range | 1-166 | 4-354 | 2-187 | 4-141 | 1-117 | 3-223 | 1-273 | 1-354 |
| Baseline platelet count, × 109/L | ||||||||
| Median | 11.5 | 22.3 | 13.3 | 20.5 | 19.3 | 16.0 | 18.5 | 18.5 |
| Range | 5.0-24.5 | 10.5-26.5 | 3.0-26.5 | 4.5-21.5 | 3.0-26.0 | 6.0-26.0 | 7.3-27.0 | 3.0-27.0 |
The median baseline platelet counts varied between the cohorts with the numerically highest baseline median counts in the 100-μg/kg cohort, and the lowest in the 75-μg/kg cohort. Across all cohorts, 12 patients had a baseline platelet count < 10 × 109/L. The 100-μg/kg cohort had a numerically younger group of patients compared with the other cohorts.
The median time from ITP diagnosis until screening for all patients was 21 months, spanning from a median of 5 months (125-μg/kg cohort) to a median of 68 months (75-μg/kg cohort).
The most frequently used ITP-related medications before enrolment were steroids (87%). IVIg had been used by 30% of the patients, and 13% had used other immunosuppressants, such as azathioprine. None of the patients had been treated with anti-D.
Safety
The dose escalation was suspended after reaching the 300-μg/kg level after evaluation of safety and efficacy data by an Independent Data Monitoring Committee.
Forty-five patients reported a total of 198 AEs during the trial (Table 2). Approximately 75% of the events (149 of 198) were assessed to be mild in intensity. Eighty events were assessed as related to rozrolimupab treatment. No AEs led to withdrawal from trial, and no deaths were recorded.
Adverse events reported in > 5% of patients treated with a single dose of rozrolimupab
| Adverse events . | Adverse event grading . | |||
|---|---|---|---|---|
| Mild . | Moderate . | Severe . | All . | |
| Headache | 13.1 | 4.9 | 1.6 | 19.7 |
| Pyrexia | 9.8 | 3.3 | 0.0 | 13.1 |
| Petechiae | 8.2 | 0.0 | 1.6 | 9.8 |
| Hematoma | 9.8 | 0.0 | 0.0 | 9.8 |
| Low platelet counts | 1.6 | 6.6 | 1.6 | 9.8 |
| Chills | 9.8 | 0.0 | 0.0 | 9.8 |
| Fatigue | 6.6 | 1.6 | 0.0 | 8.2 |
| Increased D-dimer | 4.9 | 3.3 | 0.0 | 8.2 |
| Decreased hemoglobin | 3.3 | 3.3 | 0.0 | 6.6 |
| Thrombocytopenia | 0.0 | 1.6 | 4.9 | 6.6 |
| Hematuria | 4.9 | 1.6 | 0.0 | 6.6 |
| Asthenia | 4.9 | 1.6 | 0.0 | 6.6 |
| Anemia | 3.3 | 3.3 | 0.0 | 6.6 |
| Dizziness | 6.6 | 0.0 | 0.0 | 6.6 |
| Adverse events . | Adverse event grading . | |||
|---|---|---|---|---|
| Mild . | Moderate . | Severe . | All . | |
| Headache | 13.1 | 4.9 | 1.6 | 19.7 |
| Pyrexia | 9.8 | 3.3 | 0.0 | 13.1 |
| Petechiae | 8.2 | 0.0 | 1.6 | 9.8 |
| Hematoma | 9.8 | 0.0 | 0.0 | 9.8 |
| Low platelet counts | 1.6 | 6.6 | 1.6 | 9.8 |
| Chills | 9.8 | 0.0 | 0.0 | 9.8 |
| Fatigue | 6.6 | 1.6 | 0.0 | 8.2 |
| Increased D-dimer | 4.9 | 3.3 | 0.0 | 8.2 |
| Decreased hemoglobin | 3.3 | 3.3 | 0.0 | 6.6 |
| Thrombocytopenia | 0.0 | 1.6 | 4.9 | 6.6 |
| Hematuria | 4.9 | 1.6 | 0.0 | 6.6 |
| Asthenia | 4.9 | 1.6 | 0.0 | 6.6 |
| Anemia | 3.3 | 3.3 | 0.0 | 6.6 |
| Dizziness | 6.6 | 0.0 | 0.0 | 6.6 |
Values are percentages.
Headache was the most frequently reported event with 12 patients reporting 16 events; 10 events were mild, 5 were moderate, and 1 was severe in intensity (Table 2). The majority of the events resolved on the day of onset and was not treated. The single case of severe headache was treated with paracetamol and had a duration of 2.5 hours. The longest duration of headache was 20 days for an event of moderate severity, which was treated with paracetamol. Eleven of the 16 events of headache, including 3 of the moderate events and the severe event, were reported in the 75- or 100-μg/kg cohorts.
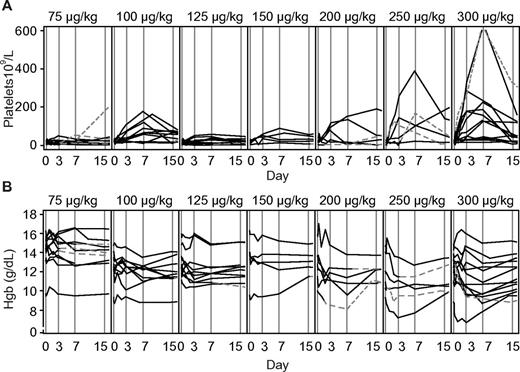
A reduction in hemoglobin concentration was observed in the majority of patients (Figure 1B) with a trend toward larger reductions with increasing doses. All patients were direct Coombs positive when tested 24 hours after treatment with rozrolimupab and remained positive at 6 weeks, except for 1 patient in each of the 250- and 300-μg/kg cohorts, respectively, who returned to negativity at 4 weeks and 6 weeks after treatment, respectively. A fall in hemoglobin concentration > 2.5 g/dL was observed at 72 hours and 7 days after treatment in 11 (18%) and 15 (25%) of the patients, respectively. No patient had a fall in hemoglobin concentration > 5 g/dL. In the dose group of 300 μg/kg, the hemoglobin concentration returned to baseline in 8 of the 13 patients in a median of 28 days (range, 1-43 days); none of the 13 patients received blood transfusions. In the remaining dose cohorts, 1 patient received a blood transfusion on the day of treatment and 3 other patients received 1 or more blood transfusions after treatment.
Effect of dose escalation of rozrolimupab on platelet and hemoglobin concentrations. Platelet responses (A) and corresponding values of hemoglobin in individual patients (B) are shown. Continuous lines indicate patients treated without rescue medication; and dashed lines, patients who received rescue medication.
Effect of dose escalation of rozrolimupab on platelet and hemoglobin concentrations. Platelet responses (A) and corresponding values of hemoglobin in individual patients (B) are shown. Continuous lines indicate patients treated without rescue medication; and dashed lines, patients who received rescue medication.
Increases in reticulocyte counts were observed in 1 of 6 patients in the 200-μg/kg cohort, 3 of 6 patients in the 250-μg/kg cohort, and in 5 of 13 patients in the 300-μg/kg cohort, all of whom also had lowering of their haptoblobin levels. Reduction in haptoglobin without concomitant increase in reticulocytes was also seen in 3 patients in the 100-μg/kg cohort and in additional patients in the 200-, 250-, and 300-μg/kg cohorts. On the day of treatment, 11 patients reported 19 events of fever and/or 6 events of chills. For a 12th patient, an infusion reaction consisting of chills and rigors was reported. Elevation of concentrations of IL-6, IL-10, MCP-1, and TNF-α after infusion with rozrolimupab was observed across all dose cohorts, and median levels peaked 2-24 hours after treatment with return to baseline levels by 2-7 days after treatment (data not shown).
In the 200- to 300-μg/kg cohorts, the FcγIIA-131HH, -HR, and -RR genotypes were found in 9, 14, and 4 patients, respectively. The FcγIIIA-158FF, -VF, and -VV genotypes were demonstrated in 6, 17, and 4 patients, respectively. Patients with the FcγIIA-131HH genotype had the highest peak levels of all 4 cytokines, whereas patients with FcγIIA-131RR genotype had the lowest peak levels. Patients with the FcγIIIA-158VV genotype had the highest peak levels of IL-6, IL-10, and MCP-1, whereas patients with the FcγIIIA-158FF genotype had the lowest peak values of these 3 cytokines. There was no change in the hemolytic activity as measured by CH50 at the sampled time points (screening, 5-8 hours and day 7) for the 150-, 200-, 250-, or 300-μg/kg cohorts.
Pretreatment values of D-dimer were within normal limits across all dose groups (data not shown). A transient rise in the concentration of D-dimer was seen in all cohorts in a dose-dependent manner. The increase peaked at 24 hours after treatment and returned toward baseline level by day 7 after treatment. The largest increase was seen in a patient from the 300-μg/kg cohort (baseline: 0.166 μg/mL; 24 hours: 9.41 μg/mL). In the 300-μg/kg cohort, the D-dimer values returned to baseline levels after 7 days in 5 of 13 patients. The rise in D-dimer levels was not associated with clinical symptoms or changes in the other coagulation parameters (prothrombin time, INR, fibrinogen and activated partial thromboplastin time) or the hemolysis parameters (concentrations of hemoglobin, reticulocytes, haptoglobin, bilirubin, lactate dehydrogenase, and free hemoglobin).
SAEs were recorded in 9 patients during the trial. No SAEs were recorded in the 125- or 300-μg/kg cohorts. Four of these events were assessed as related to rozrolimupab treatment: (1) a decrease in hemoglobin concentration from 14.1 to 11.0 g/dL (mild intensity) on day 8 was considered possibly related to rozrolimupab treatment, lasted for 22 days, and recovered without treatment (100-μg/kg cohort); (2) extravascular hemolysis with reduction of hemoglobin from 14.1 to 9.6 g/dL (severe intensity) with onset on day 7 was reported in 1 patient in the 200-μg/kg cohort who received 2 blood transfusions; (3) increase in D-dimer (mild in severity) with onset on the day of treatment and duration of 8 days was reported in 1 patient in the 250-μg/kg cohort; the patient had no clinical symptoms; and (4) another case of increase in D-dimer, reported as disseminated intravascular coagulation (DIC) of moderate severity, with onset on the infusion day was observed in the 250-μg/kg cohort. The D-dimer levels returned toward baseline at day 7, and the event was reported as probably related to trial drug. The event was an isolated increase in D-dimer with no clinical symptoms indicative of DIC.19
Platelet responses
Overall, there was a trend toward a dose-response with the 100-μg/kg cohort as an outlier (Figure 1A). An analysis of platelet response data by treatment groups is shown in Table 3. Across all cohorts, 21 of 61 patients (34%) met the response criterion on day 7. The highest percentages of responders were seen in the 100- and 300-μg/kg cohorts (70% and 62%, respectively). No patients from the 75-μg/kg cohort responded to the treatment. The highest median platelet counts were recorded 7 days after treatment for all patients (34 × 109/L), with the highest median values in the 300-μg/kg cohort (130 × 109/L, range 15-634 × 109/L). Six weeks after treatment, the median platelet count for all patients was 25 × 109/L. Again, the highest median platelet count after 6 weeks was recorded in the 300-μg/kg cohort (35 × 109/L). Unlike the other dose cohorts, the 300-μg/kg dose was able to induce platelet responses in both of 2 patients with baseline platelets < 10 × 109/L.
Number of patients with platelet responses on day 7 after a single dose of rozrolimupab
| . | Dose of rozrolimupab . | ||||||
|---|---|---|---|---|---|---|---|
| 75 μg/kg . | 100 μg/kg . | 125 μg/kg . | 150 μg/kg . | 200 μg/kg . | 250 μg/kg . | 300 μg/kg . | |
| N | 11 | 10 | 10 | 5 | 6 | 6 | 13 |
| Responders,* no. (%) | 0 (0.0) | 7 (70.0) | 2 (20.0) | 2 (40.0) | 2 (33.3) | 3 (50.0) | 8 (61.5) |
| . | Dose of rozrolimupab . | ||||||
|---|---|---|---|---|---|---|---|
| 75 μg/kg . | 100 μg/kg . | 125 μg/kg . | 150 μg/kg . | 200 μg/kg . | 250 μg/kg . | 300 μg/kg . | |
| N | 11 | 10 | 10 | 5 | 6 | 6 | 13 |
| Responders,* no. (%) | 0 (0.0) | 7 (70.0) | 2 (20.0) | 2 (40.0) | 2 (33.3) | 3 (50.0) | 8 (61.5) |
Only patients who did not receive rescue medication before day 8 are counted as responders.
The onset of platelet responses for the 300-μg/kg cohort is shown in Table 4. Already 5-8 hours after treatment, 23% of patients had platelet responses. The number of patients with platelet responses increased with time and peaked at 72 hours and at day 7, where 8 of 13 patients had platelet responses (62%). The median time to platelet response was 59 hours. The median duration of the platelet response in the 300-μg/kg cohort was 14 days.
Platelet responses over time for the 300-μg/kg cohort
| . | Time points . | ||||||
|---|---|---|---|---|---|---|---|
| 2 h . | 5-8 h . | 24 h . | 48 h . | 72 h . | 7 d . | 14 d . | |
| Responders,* no. (%) | 0 (0.0) | 3 (23.1) | 4 (30.8) | 6 (46.2) | 8 (61.5) | 8 (61.5) | 7 (53.8) |
| . | Time points . | ||||||
|---|---|---|---|---|---|---|---|
| 2 h . | 5-8 h . | 24 h . | 48 h . | 72 h . | 7 d . | 14 d . | |
| Responders,* no. (%) | 0 (0.0) | 3 (23.1) | 4 (30.8) | 6 (46.2) | 8 (61.5) | 8 (61.5) | 7 (53.8) |
Only patients who did not receive rescue medication before the specific time points are counted as responders.
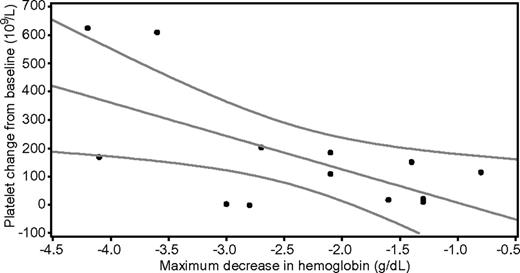
No relationship between baseline hemoglobin concentrations and platelet responses was found. For patients in the 300-μg/kg cohort, the relationship between platelet count at day 7 and maximal decrease in hemoglobin concentration is shown in Figure 2. Linear regression with platelet count as dependent variable and maximal decrease in hemoglobin concentration as predictor showed a significant correlation (P = .021; R2 = 0.40) between the absolute platelet count at day 7 and the maximal fall in hemoglobin concentration. There was also a similar relationship between the change in platelet count from baseline to day 7 and the maximal decrease in hemoglobin concentration for this cohort (P = .023; R2 = 0.39; data not shown). No apparent relationships between platelet response and FcγRIIA or FcγRIIIA genotype were found.
The difference between baseline and day 7 hemoglobin concentration (x-axis) and the corresponding values of platelet counts on day 7 (y-axis). The graph represents the fitted linear regression line with 95% confidence limits.
The difference between baseline and day 7 hemoglobin concentration (x-axis) and the corresponding values of platelet counts on day 7 (y-axis). The graph represents the fitted linear regression line with 95% confidence limits.
The majority of patients had a bleeding score of 0 or 1 during the trial. Two patients with a WHO bleeding score of 2 at entry recorded bleeding scores of 0 or 1 after treatment. Two other patients with low platelet counts at baseline recorded scores > 2 during the trial and both received rescue medication.
Twenty-one of the 61 patients (34%) used rescue medication during the trial, with IVIg and steroids (prednisolone or methylprednisolone) being the most commonly used. In the 300-μg/kg dose cohort, only 1 of 13 patients (8%) received rescue medication.
Pharmacokinetics and immunogenicity
At 30 minutes after infusion, the maximal concentration of rozrolimupab was < 1% of the expected values as calculated by the dose and plasma volume, arbitrarily set to 2.5 L, indicating that rozrolimupab was already bound to RhD+ erythrocytes. Human anti–human antibodies response of borderline magnitude was observed in a single patient.
Discussion
This dose escalation trial showed that rozrolimupab is active in previously treated patients with ITP. The 300 μg/kg was identified as the optimal dose where 62% of patients achieved platelet responses and only 1 of 13 patients needed rescue treatment. Although a selection bias cannot be excluded as all patients in this trial had responded to previous first-line treatment, this result seems comparable with platelet responses after treatment with plasma-derived anti-D7,8,20,21 and IVIg.6,22,23 Rozrolimupab demonstrated a rapid onset of effect, as 24% of patients treated with 300 μg/kg had platelet responses within 24 hours, comparable with observations after treatment with IVIg6 and anti-D at 75 μg/kg.7 This rapid increase of platelets was associated with a reduced the need for rescue medication and in the 300-μg/kg cohort, where only 1 patient (8%) received rescue medication during the trial. The rapid increase of platelets in combination with a very short infusion time of 15-20 minutes is an advantage for acute treatment of ITP in nonsplenectomized patients with ITP.
The platelet responses to plasma-derived anti-D is dose-dependent in terms of onset, magnitude, and duration,6,7 but not all nonsplenectomized patients with ITP respond to the treatment.6,19-21,24 Response rates are generally high in children and in young adults20 and generally lower in patients with pretreatment platelet levels < 10 × 109/L.6,24 The final dose of rozrolimupab (300 μg/kg) was established after dose escalation showing both a trend toward dose-dependent platelet response (except for the 100-μg/kg cohort) and a trend toward trend toward larger reductions of hemoglobin with increasing doses. In the 100-μg/kg cohort, the baseline platelet counts were numerically higher and patients were younger compared with other cohorts. Both factors may have contributed to the observed response rate of 70% at day 7. It is of note that the dose of 300 μg/kg had a higher median platelet count on day 7 than the other doses and elicited platelet responses in patients with pretreatment platelet counts < 10 × 109/L.
In this study, baseline hemoglobin levels did not correlate with platelet responses, contrary to the findings of Scaradavou et al.24 In this study, a significant correlation between the maximal fall in hemoglobin concentration and the absolute platelet count at day 7 was found for patients in the 300-μg/kg cohort. A similar correlation was observed comparing the maximal reduction in hemoglobin concentration and the change of platelets from baseline to day 7. If this correlation would be confirmed in a larger trial, it may be possible to use the magnitude of the fall in hemoglobin concentration as a predictor of response. During treatment with plasma-derived anti-D, treatment doses of even 100 μg/kg may be beneficial in selected patients with absence of platelet responses at lower doses.25 Further studies are needed to determine the therapeutic value of repeated rozrolimupab treatments for patients with ITP with small reductions of hemoglobin concentration and lack of platelet responses after treatment. In this study, we were not able to confirm the predictive value of the FcγIII genotypes for platelet responses as reported by Cooper et al,5 most probably because of an insufficient number of patients for this type of analysis.
The clinical value of any treatment for ITP lies in the rapidity and duration of platelet concentration rise. Early onset and duration of platelet response are important benchmarks for therapy with anti-D. In this study, the median duration of response after treatment with rozrolimupab was 2 weeks for the 300-μg/kg cohort. This observation is comparable with results obtained in trials with IVIg.6,22,23 Most studies with anti-D reported an average response duration of 3 weeks, although with large variations.7,20,21,24 However, a randomized study is needed to evaluate the duration of response after treatment with rozrolimupab compared with IVIg or anti-D.
The safety profile of rozrolimupab was favorable. Approximately 75% of patients reported 1 or more AEs during the trial, but 75% of these 198 events were mild in intensity. It is of note that only 20% of patients reported headaches after rozrolimupab treatment. Most of the 16 events of headache were reported in the 75- or 100-μg/kg cohorts, including 3 events of moderate intensity and 1 of severe intensity. The majority of the headaches resolved on the day of onset with little or no treatment. However, 1 patient had a persistent headache for 20 days (treated with paracetamol) and another had intermittent headaches over an 8-day period (not treated). The prevalence (20%) and short duration of these headaches need confirmation in a randomized trial that include patients with ITP treated with IVIg or anti-D.
Infusion reactions may occur during treatment with anti-D.6 In this study, medications to alleviate possible infusion-related events before the infusion of rozrolimupab were not planned per protocol. Two events of pyrexia were reported in the same patient from the 75-μg/kg cohort. All other treatment-related events of fever or chills were reported in the 200-, 250-, or 300-μg/kg cohorts. All events were resolved on the day of onset, with the exception of one event of pyrexia, which resolved after 33 hours. Fever and chills coincided with the cytokine release that peaked 2-24 hours after the infusion. The observation of maximal levels of IL-6, IL-10, and MCP-1 among patients with the FcγIIIA-158VV genotypes is in accordance with previous findings.6
Based on the mechanism of action, some degree of extravascular hemolysis is an expected outcome of treatment with rozrolimupab. Approximately one-fourth of the patients had a fall in hemoglobin concentration > 2.5 g/dL, but dramatic changes > 5.0 g/dL were not observed during the dose escalation. In the highest dose group (300 μg/kg), the hemoglobin concentration returned to baseline in most patients within 28 days, and in this dose cohort no blood transfusions were given. These observations are in line with the reduction in hemoglobin concentration seen in other trials of anti-D for ITP at 50-75 μg/kg.7,20,21,24
Acute intravascular hemolysis and DIC have been described as serious complications of anti-D treatment.26 It has been hypothesized that antibodies among anti-D preparations binding to blood groups other than RhD may play a pathogenetic role for these events.27 With rozrolimupab, containing only recombinant monoclonal antibodies specific to RhD, binding to antigens other than RhD on the erythrocytes can be ruled out. Careful analysis of such cases of intravascular hemolysis and DIC has, however, also pointed to comorbidities among patients with ITP as risk factors for the development of severe hemolysis and DIC.26-28 In this study, frequent monitoring of coagulation parameters was carried out, and a transient increase in D-dimer was observed across all dose cohorts. This increase in D-dimer was not accompanied by clinical symptoms or changes in other measured coagulation factors. A recent randomized study in patients with ITP did not show changes in D-dimer levels after treatment with IVIg and methylprednisolone, where patients in the methylprednisolone treatment arm had low levels of protein S, protein C, and antithrombin III that normalized after therapy.29 Similar changes of low levels of protein S and protein C after treatment with methylprednisolone for ITP were demonstrated in another recent report.30 The demonstrated changes in D-dimer levels observed in the present study may represent compensatory mechanisms to maintain hemostasis, which merit further exploration in future trials.
In conclusion, rozrolimupab is effective in the treatment of ITP at a dose of 300 μg/kg and exhibits a favorable safety profile at all tested dose levels. Rozrolimupab has a rapid effect on platelet levels that is sustained for a median of 14 days. Further randomized studies are needed to compare the safety and efficacy of rozrolimupab with current treatment modalities for ITP, such as plasma-derived anti-D and IVIg.
Preliminary results of this study were presented at the 52nd Annual Meeting of the American Society of Hematology, San Diego, CA, December 12, 2011.
There is an Inside Blood commentary on this article in this issue.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Jeff Craven, medical writer at Larix A/S, who assisted in writing the remaining parts of the manuscript; as well as the following collaborators who contributed to this study: Medical University of Lodz and Copernicus Memorial Hospital, Lodz, Poland: K. Chojnowski, M. Tybura; Instytut Hematologii i Transfuzjologii, Warsaw, Poland: S. Jurek, A. Sikorska; Werlhof Institut, Hannover, Germany: T. Höbbel-Schnell, St Johannes Hospital, Duisburg, Germany: M. Heinsch; Department of Hematology, Cliniques Universitaires UCL de Mont-Godinne, Yvoir, Belgium: A. Bosly, C. Chartelain, C. Graux, A. Sonet, F. Desquesnes; Department of Hematology, UZ Leuven, Belgium: J. Maertens, G. Verhoef, T. Devos, M. Delforge, D. Dierickx, S. Meers; Hospital la Paz, Madrid, Spain: V. Jiménez-Yuste, M. Martin, M. G. Salvatierra; Hospital La Fé, Valencia, Spain: J. Palau, L. Senent; Hospital General Universitario Gregorio Marañón, Madrid, Spain: A. M. R. Huerta, C. P. Izquierdo; Department of Hematology and Transplantology Medical University of Gdańsk, Poland: W. Prerjzner, M. Szarajko; Katedra i Klinika Hematologii, Onkologii i Chorob, Warsaw, Poland: J. Niesiobedzka-Krezel, M. Ziarkiewicz; Klinika Hematologii Nowotworów Krwi i Transplantologii Szpiku, Wroclow, Poland: M. Podolak-Dawidziak, I. Prajs, D. Urbaniak-Kujda; Clinic for Hematology and Clinical Immunology, Nis, Serbia: I. Cojbasic, V. Nikolic; Clinical Center Zemun, Department for Hematology, Zemun, Serbia, Z. Cvetkovic, A. Ivanovic, A. Novkovic, Z. Petrovic; “Prof Dr Ion Chiricuta” Oncology Institute Hematology Clinic, Cluj-Napoca, Romania: A. Bojan, A. Vasilache; Brasov Country Hospital, Romania: M. Lazaroiu, Davidoff Center; Beilinson Hospital, Petach Tikva, Israel: A. Inbal, O. Bairey; Bnai-Zion Medical Center, Hematology Department, Haifa, Israel: R. Laor, L. Schliamser; Saint Petersburg State Institution of Healthcare, Russia: N. Ilina, I. Samuskevich, T. Shelkovskaya; City Hospital #9, Department of Blood Systems Diseases, Kiev, Ukraine: V. Mnyshenko, N. Tretyak; Gusak Academy of AMS Ukraine, Donetsk, Ukraine: I. Lozhechnyk, S. Starchenko; Department of Hematology, Catherine Lewis Center, Hammersmith Hospital, London, United Kingdom: D. Marin; Newcastle Biomedicine Clinical Research Platforms Level 6, Newcastle On Tyne, United Kingdom: J. Hanley; Kasturba Medical College Hospital Manipal Center for Clinical Research, Karnataka, India: Y. Rao, M. Varma, S. Vidyasagar; Apollo Hospital AHERF Clinical Trial Unit, Andra Pradesh, India: P. Sripada; Aysha Hospital Pvt Ltd, Chennai, India: Shabana; Swedish Orphan Biovitrum AB, Stockholm, Sweden: M. Wiken, P. Hjälmström; and Symphogen A/S, Lyngby, Denmark: U. Hansen.
Authorship
Contribution: T.R., J.W., J.T., M.v.D.P., A.G., C.D., A.J., M.T.Á.-R., I.J., J.L., G.P.R., A.H., W.W.J., K.K., L.M.G., D.C., A.C., E.G., M.L., O.S., D.A., E.K., K.S., K.V., N.C., K.T., M.P., P.S., and T.P.R.B. identified and treated patients; H.N., N.J.Ø.S., T.P.F., and P.S.A. developed analytical methods and assays to characterize the rozrolimupab drug product; and T.R., M.F.F., and J.P. planned the clinical trial, analyzed the data, and wrote major parts of the manuscript.
Conflict-of-interest disclosure: The investigators received payments to cover trial-related expenses. H.N., N.J.Ø.S., T.P.F., P.S.A., M.F.F., and J.P. are employees of Symphogen A/S and have received stock warrants. The remaining authors declare no competing financial interests.
Correspondence: Tadeusz Robak, Department of Hematology, Medical University of Lodz, 93-513 Lodz, Pabianicka 62, Poland; e-mail: robaktad@csk.umed.lodz.pl.