Abstract
Hypoxia-inducible factors (HIFs) regulate hematopoiesis in the embryo and maintain hematopoietic stem cell function in the adult. How hypoxia and HIFs contribute to hematopoietic lineage differentiation in the adult is ill defined. Here we provide evidence that HIF-1 limits differentiation of precursors into plasmacytoid dendritic cells (pDCs). Low oxygen up-regulated inhibitor of DNA binding 2 (ID2) and suppressed Flt3-L–induced differentiation of bone marrow cells to pDCs in wild-type but not HIF-1αfl/fl LysM-Cre bone marrow cells. Moreover, pDC differentiated normally in hypoxic ID2−/− bone marrow cultures. Finally, we observed elevated pDC frequencies in bone marrow, blood, and spleen of HIF-1αfl/fl LysM-Cre and ID2−/−, but not HIF-2αfl/fl LysM-Cre mice. Our data indicate that the low oxygen content in the bone marrow might limit pDC development. This might be an environmental mechanism to restrict the numbers of these potentially autoreactive cells.
Introduction
Mammalian hematopoiesis in the BM is regulated among others by the oxygen (O2) availability. O2 concentrations in the BM range from anoxia to 6% opposed to 4%-14% in well-oxygenated tissues, including the blood.1,2 Recent data indicate that O2 gradients within the BM participate in keeping hematopoietic stem cells (HSCs) in a low-replicating pluripotent state. HSCs are located in an extremely hypoxic niche as demonstrated by dye-perfusion and engraftment studies.3,4
Hypoxia-inducible factors 1-3 (HIF-1–HIF-3) are stabilized by a low pO2 to induce adaptive gene expression. They are heterodimers consisting of distinct O2-sensitive α-subunits and a stable common β-subunit, also known as aryl hydrocarbon receptor nuclear translocator (ARNT).5 HIF-1 and HIF-2 were recently connected to HSC biology. In their hypoxic niche, HIF-1 maintains HSC quiescence,6 whereas HIF-2 maintains their self-renewing capacity.7 HIF-1 also affects embryonic hematopoiesis as demonstrated by defective myeloid and erythroid progenitor formation in HIF-1α−/− as well as ARNT−/− embryos.8 In adult hematopoiesis, HIF-1 is essential for B-cell progenitor proliferation and mature B-cell subclass differentiation.9 However, its involvement in mononuclear phagocyte development is unknown. Previous findings indicated defective development of human plasmacytoid dendritic cells (pDCs) under hypoxia in vitro.10 Therefore, we asked whether HIF-1 regulates DC lineage differentiation in mice.
Methods
Animals
HIF-1αfl/fl or HIF-2αfl/fl mice11,12 were bred with LysM-Cre transgenic mice13 in the C57BL/6 background. Age-matched C57BL/6 wild-type (WT) mice were controls. ID2−/− mice and their respective WT control were in the NMRI background.14 The guidelines of the Hessian animal care and use committee were followed.
DC generation from BM
For DC generation in vitro, 2 × 106 total BM cells/mL in RPMI 1640 with 10% FCS and 200 ng/mL recombinant murine fms-related tyrosine kinase 3-ligand (Flt3-L, PeproTech) were cultured in 6-well Ultra-Low attachment plates (Corning) for up to 9 days15 at various O2 levels as indicated, using a InVivo2 400 hypoxia workstation (Ruskinn Technologies). Alternatively, cells were cultured with 100μM dimethyloxallyl glycine (DMOG, from Biomol). Sorted monocyte/DC progenitors/common DC progenitors (MDPs/CDPs; 104 cells/well) were cultured with 200 ng/mL Flt3-L in 24-well Ultra-Low attachment plates.
Flow cytometry and cell sorting
BM cells, spleen, or whole blood cells were stained with fluorochrome-conjugated antibodies and analyzed on a LSRII/Fortessa flow cytometer (BD Biosciences). MDP/CDP were sorted from lineage− cell-enriched BM (lineage cell depletion kit and AutoMACS cell separator from Miltenyi Biotec) using a FACSAria III cell sorter (BD Biosciences). For details (antibodies, surface markers, intracellular transcription factor staining procedures), see supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). pDCs were isolated from whole BM and spleen using the pDC isolation kit and the AutoMACS cell separator (Miltenyi Biotec). Purity of spleen pDCs was > 95%; CD11b+ cells were absent. BM pDCs were further enriched for CD11b− SiglecH+ cells using FACS sorting to achieve > 95% purity.
IFN-α ELISA
Murine IFN-α in supernatants of 10 μg/mL CpG-A (InvivoGen)-stimulated pDCs was quantified using VeriKine ELISA (PBL InterferonSource).
Quantitative PCR
Quantitative PCR was performed as described.10 Primer sequences for PU.1, β-actin, TATA-binding protein, and HIF-1α-exon2 are shown in supplemental Table 1. For amplifying murine E2-2/TCF4 (Mm_Tcf4_1_SG) and ID2 (Mm_Id2_1_SG) predesigned QuantiTect Primer Assays (QIAGEN) were used. Results were analyzed using Gene Expression Macro (Bio-Rad). Actin and/or TATA-binding protein served as the internal controls.
Results and discussion
Hypoxia attenuated pDC generation from human monocytes, which was associated with up-regulation of inhibitor of DNA-binding 2 (ID2).10 ID2 is thought to suppress the pDC lineage-determining transcription factor E2-216 and can be up-regulated by HIF-1.17 Therefore, we questioned whether depletion of HIF-1α in DC progenitors would affect lineage commitment in vivo. We used mice lacking HIF-1α in cells expressing lysozyme 2 (LysM; HIF-1αfl/fl LysM-Cre). Although mature DCs do not express LysM, MDPs that differentiate into CDPs, which give rise to pDC and cDC,18 express LysM.19
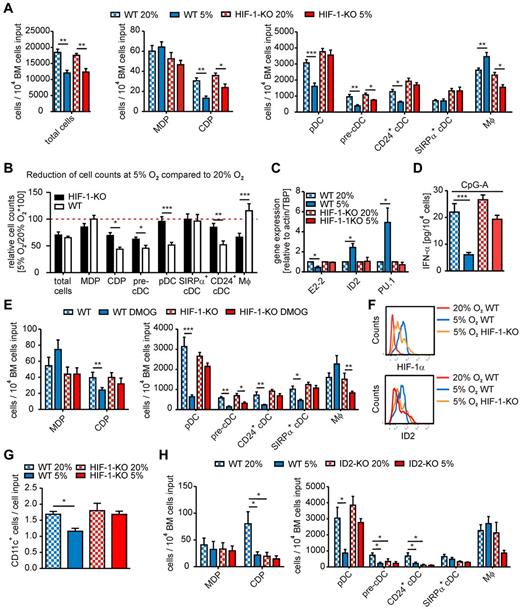
First, we cultured WT BM cell suspensions with Flt3-L at different pO2. At 20% O2, a characteristic cell population was expanded, which was blocked at pO2 levels up to 4% (supplemental Figure 1), probably because of induction of quiescence.2 At 5% O2, a cell population was generated that corresponded in size and granularity to cells expanded under 20% O2 (supplemental Figure 1B). Thus, we performed Flt3-L BM cultures of HIF-1αfl/fl LysM-Cre or WT cells at 20% (normoxia) or 5% O2 (hypoxia) and analyzed differentiation along the mononuclear phagocyte lineage using flow cytometry15,20-23 (supplemental Figure 2). Total cell numbers decreased similarly under hypoxia compared with normoxia irrespective of the genotype (Figure 1A-B). The occurrence of MDP was unaltered between experimental groups, whereas CDP and pre-cDC numbers strongly decreased under hypoxia in WT and to a lesser extent in HIF-1αfl/fl LysM-Cre cultures (Figure 1A-B). CD24+ cDC (equivalents of splenic CD8+ DC15 ) numbers only decreased in hypoxic WT cultures, whereas SIRPα+ cDC (equivalents of splenic CD8− DC15 ) were unchanged. However, hypoxia markedly reduced pDC and increased macrophage (Mφ) numbers in WT but not in HIF-1αfl/fl LysM-Cre cultures (Figure 1A-B). Changes in cell populations were mirrored by expression of lineage-determining transcription factors. In WT, but not HIF-1αfl/fl LysM-Cre cultures, expression of pDC-specific E2-216 was significantly decreased, whereas ID2 and PU.1, expressed in cDCs and Mφ,18 were elevated (Figure 1C). ID2 primers were validated using spleen cells of ID2−/− versus WT mice (supplemental Figure 3A). Changes in pDC numbers were also reflected by the production of IFN-α on CpG-A stimulation (Figure 1D). Thus, hypoxia-induced HIF-1 activation repressed the generation of DCs, predominantly pDCs, while facilitating Mφ generation (supplemental Figure 2B).
Impact of hypoxia and HIF-1α on BM cell differentiation in vitro. (A-E) BM single-cell suspensions of either WT or HIF-1αfl/fl LysM-Cre (HIF-1-KO) mice were generated. A total of 2 × 106 cells/mL were seeded in Ultra-Low attachment plates. (A-D) Cells were cultured with 200 ng/mL Flt3-L at 20% or 5% O2 for 9 days. (A,E,H) Total numbers of the indicated cell populations were determined by flow cytometry. For gating strategies, see supplemental Methods and supplemental Figure 2. (A) Data are mean ± SEM of cells cultured from 6 animals of each genotype (3 independent experiments using cells of 2 mice each). (B) Relative changes in cell counts of the indicated populations under 5% versus 20% O2 in WT or HIF-1-KO cultures were compared. The red dotted line indicates the 20% O2 control values. Data are mean ± SEM of cells cultured from 10 animals of each genotype (4 independent experiments using cells of 2 or 3 mice each). (C) Relative mRNA expression of E2-2, ID2, and PU.1 quantified by quantitative PCR is shown. mRNA levels of 20% O2 WT or HIF-1-KO were set to 1. Data are mean ± SEM of 4 independent experiments using pooled cells of 2 or 3 mice each. (D) IFN-α secreted from 104 cells in 10 μg/mL CpG-stimulated BM cultures was determined by ELISA. Data are mean ± SEM of cells cultured from 6 animals of each genotype (3 independent experiments using cells of 2 mice each). (E) Cells were cultured with 200 ng/mL Flt3-L with or without 100μM DMOG for 9 days. Data are mean ± SEM of cells cultured from 8 animals of each genotype (3 independent experiments using cells of 2 or 3 mice each). (F-G) Monocyte/DC progenitors and common DC progenitors were isolated from whole BM cell suspensions of WT or HIF-1-KO mice using untouched magnetic separation followed by FACS sorting (see supplemental Methods and supplemental Figure 4), and 104 cells/well were seeded in Ultra-Low attachment plates. Cells were cultured with Flt3-L at 20% or 5% O2 for 48 hours. (F) Intracellular expression of HIF-1α and ID2 in cultured MDPs/CDPs was quantified by flow cytometry using biotin-coupled HIF-1α and ID2 antibodies and streptavidin-PE-CF495 (see supplemental Methods and supplemental Figure 5). A representative histogram of 3 independent experiments using pooled cells of 2 or 3 mice each is shown. (G) The number of CD11c-expressing cells generated from MDPs/CDPs on 48-hour culture is shown. Data are mean ± SEM of 3 independent experiments using pooled cells of 2 or 3 mice each. (H) A total of 2 × 106 BM cells of ID2-KO and respective WT mice were cultured with 200 ng/mL Flt3-L at 20% or 5% O2 for 9 days. Data are mean ± SEM of cells cultured from 8 animals of each genotype (3 independent experiments using cells of 2 or 3 mice each). Data were analyzed using GraphPad Prism Version 5.0 for Windows. P values were calculated using Student t test (B) or 1-way ANOVA (A,C-E,G-H) with Bonferroni correction. Significant differences between experimental groups: *P < .05, **P < .01, ***P < .001.
Impact of hypoxia and HIF-1α on BM cell differentiation in vitro. (A-E) BM single-cell suspensions of either WT or HIF-1αfl/fl LysM-Cre (HIF-1-KO) mice were generated. A total of 2 × 106 cells/mL were seeded in Ultra-Low attachment plates. (A-D) Cells were cultured with 200 ng/mL Flt3-L at 20% or 5% O2 for 9 days. (A,E,H) Total numbers of the indicated cell populations were determined by flow cytometry. For gating strategies, see supplemental Methods and supplemental Figure 2. (A) Data are mean ± SEM of cells cultured from 6 animals of each genotype (3 independent experiments using cells of 2 mice each). (B) Relative changes in cell counts of the indicated populations under 5% versus 20% O2 in WT or HIF-1-KO cultures were compared. The red dotted line indicates the 20% O2 control values. Data are mean ± SEM of cells cultured from 10 animals of each genotype (4 independent experiments using cells of 2 or 3 mice each). (C) Relative mRNA expression of E2-2, ID2, and PU.1 quantified by quantitative PCR is shown. mRNA levels of 20% O2 WT or HIF-1-KO were set to 1. Data are mean ± SEM of 4 independent experiments using pooled cells of 2 or 3 mice each. (D) IFN-α secreted from 104 cells in 10 μg/mL CpG-stimulated BM cultures was determined by ELISA. Data are mean ± SEM of cells cultured from 6 animals of each genotype (3 independent experiments using cells of 2 mice each). (E) Cells were cultured with 200 ng/mL Flt3-L with or without 100μM DMOG for 9 days. Data are mean ± SEM of cells cultured from 8 animals of each genotype (3 independent experiments using cells of 2 or 3 mice each). (F-G) Monocyte/DC progenitors and common DC progenitors were isolated from whole BM cell suspensions of WT or HIF-1-KO mice using untouched magnetic separation followed by FACS sorting (see supplemental Methods and supplemental Figure 4), and 104 cells/well were seeded in Ultra-Low attachment plates. Cells were cultured with Flt3-L at 20% or 5% O2 for 48 hours. (F) Intracellular expression of HIF-1α and ID2 in cultured MDPs/CDPs was quantified by flow cytometry using biotin-coupled HIF-1α and ID2 antibodies and streptavidin-PE-CF495 (see supplemental Methods and supplemental Figure 5). A representative histogram of 3 independent experiments using pooled cells of 2 or 3 mice each is shown. (G) The number of CD11c-expressing cells generated from MDPs/CDPs on 48-hour culture is shown. Data are mean ± SEM of 3 independent experiments using pooled cells of 2 or 3 mice each. (H) A total of 2 × 106 BM cells of ID2-KO and respective WT mice were cultured with 200 ng/mL Flt3-L at 20% or 5% O2 for 9 days. Data are mean ± SEM of cells cultured from 8 animals of each genotype (3 independent experiments using cells of 2 or 3 mice each). Data were analyzed using GraphPad Prism Version 5.0 for Windows. P values were calculated using Student t test (B) or 1-way ANOVA (A,C-E,G-H) with Bonferroni correction. Significant differences between experimental groups: *P < .05, **P < .01, ***P < .001.
Culturing BM cells with 100μM of the prolyl hydroxylase inhibitor DMOG to stabilize HIF-1α under normoxic conditions recapitulated hypoxia-induced changes in Flt3-L BM cultures, which were absent in BM cells of HIF-1αfl/fl LysM-Cre mice (Figure 1E). To formally prove HIF-1α stabilization in DC progenitors at 5% O2 and its deletion in HIF-1αfl/fl LysM-Cre DC progenitors, we isolated MDP/CDP from BM (supplemental Figure 4), cultured them with Flt3-L for 48 hours at normoxia or 5% O2, determined intracellular expression of HIF-1α and ID2 by FACS (supplemental Figure 5), and followed their differentiation to CD11c+ cells. HIF-1α expression was higher at 5% O2 in WT cells but markedly reduced in HIF-1αfl/fl LysM-Cre cells (Figure 1F), confirming that LysM expression in MDP/CDP is not uniform.19 Importantly, ID2 expression followed that of HIF-1α. ID2 was expressed under normoxic conditions (compared with ID2−/− cells; supplemental Figure 3B) and culturing under 5% O2 elevated intracellular ID2 in WT, but not in HIF-1αfl/fl LysM-Cre cells (Figure 1F), suggesting a HIF-1α dependency. CD11c+ cells generated from MDP/CDP on 48-hour culture with Flt3-L were significantly suppressed under hypoxia in WT but not HIF-1αfl/fl LysM-Cre cells (Figure 1G).
To better understand ID2-dependent DC differentiation in vitro, we cultured ID2−/− compared with WT BM cells with Flt3-L at 20% or 5% O2. Hypoxia reduced CDP, pre-cDC, pDC, and CD24+ cDC numbers in WT cultures compared with normoxia (Figure 1H). Of note, the reduction of pDC numbers at 5% O2 was absent in ID2−/− cultures, supporting the hypothesis that hypoxia/HIF-1-induced ID2 suppresses pDC development. The picture, however, is more complex as CDP and pre-CDC numbers were significantly lower and CD24+ cDC were practically absent in ID2−/− cultures, even at normoxia, and were not significantly reduced at hypoxia (Figure 1H), supporting the notion that development of the CD8+ spleen cDC population (and their CD24+ in vitro counterparts) requires ID2.18 The mechanism of HIF-1–dependent suppression of CDP, pre-cDC, and CD24+ cDC generation, in a situation where expression of transcription factors required for cDC generation, such as ID2 and PU.1 is high, remains to be determined and might involve modifying the expression of cDC-specific factors. However, our data suggest that hypoxia-induced HIF-1α negatively regulates pDC differentiation in vitro by up-regulating ID2.
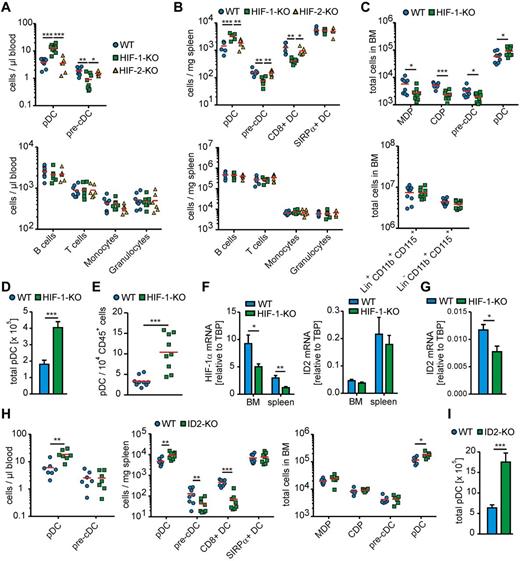
Given the low oxygen levels in the BM, which we mimicked in vitro, we asked whether deletion of HIF-1α in LysM-Cre–expressing cells affects DC development in vivo (supplemental Figure 6). pDC numbers in the blood of HIF-1αfl/fl LysM-Cre mice were markedly increased, whereas pre-cDC counts were reduced compared with WT mice (Figure 2A). DC subtype differences in the blood were mirrored in the spleen. Spleens of HIF-1αfl/fl LysM-Cre mice contained higher pDC but reduced pre-cDC numbers compared with WT mice (Figure 2B). Interestingly CD8+ splenic cDCs were reduced in HIF-1αfl/fl LysM-Cre mice, whereas SIRPα+ cDCs were unchanged. In the BM of HIF-1αfl/fl LysM-Cre mice, pDC numbers were significantly elevated, but not as dramatic as in blood and spleen, although MDPs and particularly CDPs and pre-cDCs were reduced compared with WT BM (Figure 2C). This situation might either reflect a steady-state condition where pDCs are rapidly recruited from the BM into the blood, as observed before for virus-induced pDC mobilization in macaques,24 or might be the result of pDC development from other sources, such as lymphoid tissue, as suggested earlier.25
HIF-1α and ID2, but not HIF-2α, limit pDC development in vivo. (A-D) BM, blood, and spleen of age-matched WT, HIF-1αfl/fl LysM-Cre (HIF-1-KO), or HIF-2αfl/fl LysM-Cre (HIF-2-KO) mice was analyzed by flow cytometry as indicated in supplemental Methods and supplemental Figure 6. (A) Quantification of the indicated cell populations per microliter of blood is shown. Individual data points correspond to 1 animal each (6-8 animals were analyzed in groups of 2 or 3 in 3 independent experiments. Red represents mean values. (B) Quantification of the indicated cell populations per milligram of spleen is shown. Individual data points correspond to 1 animal each (6 animals were analyzed in groups of 2 in 3 independent experiments. Red represents mean values. (C) Quantification of the indicated cell populations in the entire BM harvested from both hind limbs. Individual data points correspond to 1 animal (6 animals were analyzed in groups of 2 in 3 independent experiments. Red bars represent mean values. (D) The sum of total pDCs from BM, spleen, and peripheral blood from 6 animals of each genotype. (E) Mammary tumors were extracted from 100-day-old HIF-1αfl/fl LysM-Cre (HIF-1-KO) or WT mice expressing the PyMT oncogene. Single-cell suspensions were analyzed by polychromatic flow cytometry as indicated in supplemental Figure 6. Quantitative analysis of relative pDC amounts within the total immune cell population. Individual data points corresponding to 1 animal each (9 animals were analyzed in at least 6 independent experiments. Red bars represent mean values. (F) pDCs were isolated from spleens and BM of WT and HIF-1αfl/fl LysM-Cre mice. Spleen pDCs were isolated from single-cell suspensions using untouched magnetic sorting and BM pDCs by untouched magnetic sorting followed by further enrichment using FACS sorting. Relative mRNA expression of exon 2 of the HIF-1α gene transcript and of ID2 quantified by quantitative PCR. pDCs of 6 animals of each genotype were analyzed in groups of 2 in 3 independent experiments. (G) MDPs and CDPs were isolated from whole BM of WT or HIF-1-KO mice (see supplemental Methods and supplemental Figure 4), pooled and analyzed for relative ID2 mRNA expression. MDPs/CDPs of 6 animals of each genotype were analyzed in groups of 2 in 3 independent experiments. (H) BM, blood, and spleen of age-matched ID2−/− (ID2-KO) mice and the respective WT control animals were analyzed by flow cytometry as indicated in supplemental Methods and supplemental Figure 6. Quantification of the indicated cell populations per microliter of blood, per milligram of spleen and in the entire hind limb BM. Individual data points correspond to 1 animal (6-8 animals were analyzed in groups of 2 or 3 in 3 independent experiments. Red bars represent mean values. (I) The sum of total pDCs from BM, spleen, and peripheral blood from 6 WT or ID2-KO. Data were analyzed using GraphPad Prism Version 5.0 for Windows. P values were calculated using Student t test (C-E, G-I) or 1-way ANOVA (A,B,F) with Bonferroni correction. Significant differences between experimental groups: *P < .05, **P < .01, ***P < .001.
HIF-1α and ID2, but not HIF-2α, limit pDC development in vivo. (A-D) BM, blood, and spleen of age-matched WT, HIF-1αfl/fl LysM-Cre (HIF-1-KO), or HIF-2αfl/fl LysM-Cre (HIF-2-KO) mice was analyzed by flow cytometry as indicated in supplemental Methods and supplemental Figure 6. (A) Quantification of the indicated cell populations per microliter of blood is shown. Individual data points correspond to 1 animal each (6-8 animals were analyzed in groups of 2 or 3 in 3 independent experiments. Red represents mean values. (B) Quantification of the indicated cell populations per milligram of spleen is shown. Individual data points correspond to 1 animal each (6 animals were analyzed in groups of 2 in 3 independent experiments. Red represents mean values. (C) Quantification of the indicated cell populations in the entire BM harvested from both hind limbs. Individual data points correspond to 1 animal (6 animals were analyzed in groups of 2 in 3 independent experiments. Red bars represent mean values. (D) The sum of total pDCs from BM, spleen, and peripheral blood from 6 animals of each genotype. (E) Mammary tumors were extracted from 100-day-old HIF-1αfl/fl LysM-Cre (HIF-1-KO) or WT mice expressing the PyMT oncogene. Single-cell suspensions were analyzed by polychromatic flow cytometry as indicated in supplemental Figure 6. Quantitative analysis of relative pDC amounts within the total immune cell population. Individual data points corresponding to 1 animal each (9 animals were analyzed in at least 6 independent experiments. Red bars represent mean values. (F) pDCs were isolated from spleens and BM of WT and HIF-1αfl/fl LysM-Cre mice. Spleen pDCs were isolated from single-cell suspensions using untouched magnetic sorting and BM pDCs by untouched magnetic sorting followed by further enrichment using FACS sorting. Relative mRNA expression of exon 2 of the HIF-1α gene transcript and of ID2 quantified by quantitative PCR. pDCs of 6 animals of each genotype were analyzed in groups of 2 in 3 independent experiments. (G) MDPs and CDPs were isolated from whole BM of WT or HIF-1-KO mice (see supplemental Methods and supplemental Figure 4), pooled and analyzed for relative ID2 mRNA expression. MDPs/CDPs of 6 animals of each genotype were analyzed in groups of 2 in 3 independent experiments. (H) BM, blood, and spleen of age-matched ID2−/− (ID2-KO) mice and the respective WT control animals were analyzed by flow cytometry as indicated in supplemental Methods and supplemental Figure 6. Quantification of the indicated cell populations per microliter of blood, per milligram of spleen and in the entire hind limb BM. Individual data points correspond to 1 animal (6-8 animals were analyzed in groups of 2 or 3 in 3 independent experiments. Red bars represent mean values. (I) The sum of total pDCs from BM, spleen, and peripheral blood from 6 WT or ID2-KO. Data were analyzed using GraphPad Prism Version 5.0 for Windows. P values were calculated using Student t test (C-E, G-I) or 1-way ANOVA (A,B,F) with Bonferroni correction. Significant differences between experimental groups: *P < .05, **P < .01, ***P < .001.
Contrasting to the in vitro data, developmental changes in HIF-1αfl/fl LysM-Cre mice were DC-specific because neither monocyte counts in the BM nor any other major immune cell population in blood or spleen (Figure 2A-C) were affected. Accordingly, spleen size was unaltered between the different genotypes (supplemental Figure 7). In vitro, HIF-1 affected MDP to monocyte versus CDP differentiation, and further CDP to pDC differentiation, whereas in vivo HIF-1 repressed pDC generation from CDP, probably because of individual DC progenitor populations residing in heterogeneous environmental niches in the BM, which cannot be easily recapitulated in vitro. Importantly, changes in DC lineage development were HIF-1α–specific as immune cell counts in HIF-2αfl/fl LysM-Cre mice reflected those in WT mice (Figure 2A-B). Apparently, HIF-1α limits pDC generation in the BM. In its absence, pDC development is encouraged and pDC numbers increase (Figure 2D). This was also observed in a pathologic setting. Breast tumors of HIF-1αfl/fl LysM-Cre mice crossed into the PyMT-MMTV background showed markedly enhanced pDC frequencies compared with WT controls (Figure 2E).
The possibility remains that elevated pDC numbers in HIF-1αfl/fl LysM-Cre mice are an indirect result of, for instance, growth factor production by bystander myeloid cells expressing LysM and thus lacking HIF-1α. However, if enhanced pDC development in HIF-1αfl/fl LysM-Cre mice results from the absence of HIF-1–dependent transcriptional changes in MDPs or CDPs, pDCs developing from these progenitors should lack HIF-1α. Indeed, sorted pDCs from spleen and BM of HIF-1αfl/fl LysM-Cre mice expressed significantly lower levels of HIF-1α mRNA, specifically exon 2 (Figure 2F), which is excised on Cre-mediated recombination. Thus, a significant pDC fraction differentiates from LysM-expressing progenitors. ID2 expression in pDC from spleen and BM was unaltered (Figure 2F), indicating that mature pDCs do not face hypoxia. However, sorted MDPs/CDPs from HIF-1αfl/fl LysM-Cre mice expressed lower levels of ID2 compared with MDPs/CDPs from control animals (Figure 2G), although ID2 expression was generally low in these cells.
Previously, the lack of CD8+ DC in the spleen of ID2−/− mice was reported and a relative increase in spleen pDCs was suggested.26 We were able to reproduce these data in a quantitative manner. Strikingly, ID2−/− mice phenocopied HIF-1αfl/fl LysM-Cre mice with regard to spleen DC populations (Figure 2H). Spleen size was comparable between ID2−/− and WT mice (supplemental Figure 7). However, although pDC numbers were significantly elevated in both blood and BM of ID2−/− mice, neither MDP, nor CDP in the BM nor pre-cDCs in blood and BM were affected (Figure 2H-I). These data may support the notion that pDC development in the BM is suppressed by ID2, which is under the control of HIF-1.
Our data point to HIF-1α as a negative regulator of murine pDC differentiation. This probably demands HIF-1–facilitated ID2 induction as suggested by the in vitro data, which in turn might repress E2-2.16 Based on our in vitro data, hypoxia might be driving HIF-1 accumulation, although we cannot exclude that other HIF-1–activating mechanisms contribute in vivo, such as redox modifications or inflammatory cytokine production.5 Nevertheless, our findings suggest the intriguing possibility that hypoxia sensitivity of pDC development might have evolved to restrict the numbers of potentially highly autoreactive cells.27 Perspectively, hypoxia/HIF-1–dependent generation of distinct DC populations might be of importance in stress-induced extramedullary hematopoiesis. Finally, hypoxia might not only control pDC development as E2-2 also determines mature pDC function.28
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank R. S. Johnson (University of Cambridge, Cambridge, United Kingdom) for the HIF-1αfl/fl mice, M. C. Simon (University of Pennsylvania, Philadelphia, PA) for the HIF-2αfl/fl mice, D. N. Müller (MDC Berlin, Germany) and Y. Yokota (Fukui Medical University, Fukui, Japan) for the ID2−/− mice, T. Reinheckel (University of Freiburg, Freiburg, Germany) for the MMTV-PyMT mice, and Franz-Josef Streb and Praveen Mathoor for excellent technical assistance.
This work was supported by Deutsche Forschungsgemeinschaft (BR999, SFB815, ECCPS) and Deutsche Krebshilfe.
Authorship
Contribution: B.W., A.W., D.S., W.S., C.H., J.M., S.L., and S.E. performed experiments; A.W., B.W., D.S., W.S., N.D., and B.B. designed the overall research; A.W. analyzed the data; and A.W. and B.B. wrote the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Bernhard Brüne, Goethe-University Frankfurt, Institute of Biochemistry I-Pathobiochemistry, Theodor-Stern-Kai 7, 60590 Frankfurt, Germany; e-mail: bruene@pathobiochemie1.de; and Andreas Weigert, Goethe-University Frankfurt, Institute of Biochemistry I-Pathobiochemistry, Theodor-Stern-Kai 7, 60590 Frankfurt, Germany; e-mail: weigert@zbc.kgu.de.
References
Author notes
A.W. and B.W. contributed equally to this study.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal