Abstract
The karyotype is so far the most important prognostic parameter in acute myeloid leukemia (AML). Molecular mutations have been analyzed to subdivide AML with normal karyotype into prognostic subsets. The aim of this study was to develop a prognostic model for the entire AML cohort solely based on molecular markers. One thousand patients with cytogenetic data were investigated for the following molecular alterations: PML-RARA, RUNX1-RUNX1T1, CBFB-MYH11, FLT3-ITD, and MLL-PTD, as well as mutations in NPM1, CEPBA, RUNX1, ASXL1, and TP53. Clinical data were available in 841 patients. Based on Cox regression and Kaplan-Meier analyses, 5 distinct prognostic subgroups were identified: (1) very favorable: PML-RARA rearrangement (n = 29) or CEPBA double mutations (n = 42; overall survival [OS] at 3 years: 82.9%); (2) favorable: RUNX1-RUNX1T1 (n = 35), CBFB-MYH11 (n = 31), or NPM1 mutation without FLT3-ITD (n = 186; OS at 3 years: 62.6%); (3) intermediate: none of the mutations leading to assignment into groups 1, 2, 4, or 5 (n = 235; OS at 3 years: 44.2%); (4) unfavorable: MLL-PTD and/or RUNX1 mutation and/or ASXL1 mutation (n = 203; OS at 3 years: 21.9%); and (5) very unfavorable: TP53 mutation (n = 80; OS at 3 years: 0%; P < .001). This comprehensive molecular characterization provides a more powerful model for prognostication than cytogenetics.
Introduction
The karyotype of the leukemic blasts determined by chromosome banding analysis is accepted to be the most important prognostic parameter in acute myeloid leukemia (AML). The cytogenetic favorable subset includes cases with t(15;17)(q22;q12) leading to the molecular PML-RARA rearrangement. Patients with t(8;21)(q22;q22)/RUNX1-RUNX1T1 and inv(16)(p13q22)/t(16;16)(p13;q22)/CBFB-MYH11—the so-called CBF leukemias—are also associated with favorable outcome.1-3 On the other hand, −5/5q−, −7/7q−, −17/abn17p, inv(3)(q21q26)/t(3;3)(q21;q26) alterations, as well as complex karyotypes, are associated with poor prognosis.1-4 Importantly, approximately 20%-30% of AML show rare cytogenetic abnormalities and therefore their prognostic impact can only be analyzed in very large datasets.3 In addition, approximately 45% of AML cases have a normal karyotype (CN-AML) at diagnosis and cannot be further subdivided based on cytogenetics. Although showing strong clinical heterogeneity, these patients are currently assigned to the intermediate-risk cytogenetic prognostic group.3 Recently, the evaluation of molecular mutations (ie, mutations in NPM1, CEBPA, RUNX1, ASXL1, FLT3-ITD, and MLL-PTD) proved valuable for subdividing CN-AML into subsets with different outcome.5-13 Furthermore, using novel technologies such as sequencing studies of whole genomes or whole exomes, further mutations in a variety of genes such as IDH1, IDH2, DNMT3A, TET2, and BCOR were described in AML.14-17 In addition, prognostic classification models have aimed at combining cytogenetics and molecular genetic biomarkers.18,19 These proposals were based on literature reviews, and evaluation in large datasets has just begun. In a recent study, we have shown that the attractive model proposed by the European LeukemiaNet (ELN) could even be refined.20
In past years, the laboratory methodologies have been improved and sequencing can now be widely used in routine diagnostics.21,22 Conventional karyotyping which relies on analyses of viable leukemia cells, optimal cultivation conditions, and experienced personnel still represents a labor-intensive and time-consuming methodology. In comparison, molecular characterization of AML based on RT-PCR and genomic DNA sequencing analyses is less challenging to be established and maintained as a routine technique, and further can be very efficiently performed in large sets of patients. It also has increasing value to measure minimal residual disease (MRD).
The aim of this study was to substitute the cytogenetic-based prognostic model in AML by investigating the power of gene mutation analysis for prognostication in a comprehensive cohort comprising all cytogenetically defined AML subsets. Molecular markers, which already have shown to identify prognostic subsets among patients with normal karyotype, were studied. In addition, TP53 mutation analysis was included for the first time in the total cohort of AML, based on the hypothesis that this biomarker might identify the AML subset with the worst outcome as we and others have shown that TP53 mutations are very frequent in AML with complex karyotype.23,24
Methods
Patient cohort
One thousand bone marrow (n = 841) or peripheral blood (n = 159) samples obtained from patients with AML at time of diagnosis were sent to the MLL Munich Leukemia Laboratory between August 2005 and May 2011 for diagnostic assessment. Clinical follow-up data were available in 841 cases and was the basis for survival analyses. Median follow-up time was 23.7 months. All patients were treated with AML-specific intensive treatment protocols comprising 1 or 2 courses of induction therapy with standard-dose or high-dose cytarabine and an anthracycline as well as at least 1 course of consolidation therapy of identical intensity.7,25,26 Allogeneic stem cell transplantation was performed in 149 patients. Patients gave informed consent to laboratory analyses and to the use of data records for research purposes. The study abides by the rules of the local internal review board and the tenets of the revised Helsinki protocol. Regarding the patients, 463 were female and 537 were male; the median age in the total cohort was 66.8 years (range, 3.4-100.4). Three patients were 17 years or younger, 352 patients 18 to 60 years old, and 645 patients older than 60 years. Detailed information on the age distribution is provided in supplemental Figure 1 (available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
The complete cohort was composed of: t(15;17)(q22;q12), n = 33; t(8;21)(q22;q22), n = 40; inv(16)(p13q22), n = 30; normal karyotype, n = 449; complex karyotype (defined as ≥ 4 unrelated abnormalities),3 n = 116; other abnormalities, n = 332. Of note, in 2 cases with a normal karyotype and 1 case with an aberrant karyotype, a cytogenetically cryptic CBFB-MYH11 rearrangement was detectable resulting in a total of 33 cases with CBFB-MYH11 rearrangement. Thus, for all evaluations, the subset with normal karyotypes comprises 447 patients and the subset with other abnormalities 331 patients.
Cytogenetics and molecular mutation screening
Chromosome banding followed previously described methods.27 All cases with available material (n = 858) were retrospectively analyzed by fluorescence in situ hybridization (FISH) on interphase nuclei for TP53 deletions as described.28 Molecular analyses for FLT3-ITD (internal tandem duplication),8 MLL-PTD (partial tandem duplication),29 and mutations of NPM1 were performed as described.9,30-33 The complete regions of CEBPA and RUNX1 were investigated by 454 deep sequencing (Roche Applied Science) as previously described.34,35 Furthermore, ASXL1 exon 12 aberrations were investigated by Sanger sequencing.33 The controversial ASXL1 variant p.Gly646TrpfsX12 was scored as a mutation as we and others have convincing data that this is a somatic mutation (for further details, see supplemental Methods). Mutations were compared with dbSNP (Version 135; http://www.ncbi.nlm.nih.gov/projects/SNP/) to filter out common polymorphisms.
Within the favorable cytogenetic risk group, not all above-mentioned molecular markers were analyzed in the whole subset because the hierarchical model prognostic assignment was based on the detection of the specific fusion transcripts irrespective of further mutations (see “Prognostic model based on molecular markers only”). The number of cases analyzed for the respective mutations are depicted in Table 1.
Frequency of molecular mutations in distinct cytogenetic risk groups
| . | Favorable . | Intermediate . | Unfavorable . | ||||
|---|---|---|---|---|---|---|---|
| t(15;17)(q22;q12) PML/RARA, n = 33 (%) . | t(8;21)(q22;q22) RUNX1/RUNX1T1, n = 40 (%) . | inv(16)(p13q22) CBFB/MYH11, n = 33 (%) . | Normal karyotype, n = 447 (%) . | Intermediate-risk aberrant karyotype, n = 241 (%) . | Complex karyotype, n = 116 (%) . | Other unfavorable, n = 90 (%) . | |
| NPM1 | 0/12 | 0/36 | 0/24 | 228/447 (51.0) | 47/241 (19.5) | 4/116 (3.4) | 3/90 (3.3) |
| FLT3-ITD | 18/33 (54) | 1/39 (2.6) | 6/33 (18.2) | 95/447 (21.3) | 28/241 (11.6) | 4/116 (3.4) | 7/90 (7.8) |
| CEPBA | 2/33 (6.1) | 0/39 | 0/31 | 52/447 (11.6) | 16/241 (6.6) | 1/116 (0.9) | 4/90 (4.4) |
| CEPBA single-mut | 2/2 | 0/0 | 0/0 | 17/52 (32.7) | 8/16 (50.0) | 1/1 | 3/4 |
| CEPBA double-mut | 0/2 | 0/0 | 0/0 | 35/52 (67.3) | 8/16 (50.0) | 0/1 | 1/4 |
| MLL-PTD | 0/2 | 0/31 | 0/25 | 35/447 (7.8) | 11/241 (4.6) | 3/116 (2.6) | 8/90 (8.9) |
| ASXL1 | 0/0 | 0/39 | 0/4 | 55/447 (12.3) | 62/241 (25.7) | 9/116 (7.8) | 18/90 (20) |
| RUNX1 | 0/0 | 0/2 | 0/11 | 78/447 (17.4) | 59/241 (24.5) | 5/116 (4.3) | 20/90 (22.2) |
| TP53 | 0/33 | 1/40 (2.5) | 0/33 | 5/447 (1.1) | 7/241 (2.9) | 85/116 (73.3) | 17/90 (18.9) |
| . | Favorable . | Intermediate . | Unfavorable . | ||||
|---|---|---|---|---|---|---|---|
| t(15;17)(q22;q12) PML/RARA, n = 33 (%) . | t(8;21)(q22;q22) RUNX1/RUNX1T1, n = 40 (%) . | inv(16)(p13q22) CBFB/MYH11, n = 33 (%) . | Normal karyotype, n = 447 (%) . | Intermediate-risk aberrant karyotype, n = 241 (%) . | Complex karyotype, n = 116 (%) . | Other unfavorable, n = 90 (%) . | |
| NPM1 | 0/12 | 0/36 | 0/24 | 228/447 (51.0) | 47/241 (19.5) | 4/116 (3.4) | 3/90 (3.3) |
| FLT3-ITD | 18/33 (54) | 1/39 (2.6) | 6/33 (18.2) | 95/447 (21.3) | 28/241 (11.6) | 4/116 (3.4) | 7/90 (7.8) |
| CEPBA | 2/33 (6.1) | 0/39 | 0/31 | 52/447 (11.6) | 16/241 (6.6) | 1/116 (0.9) | 4/90 (4.4) |
| CEPBA single-mut | 2/2 | 0/0 | 0/0 | 17/52 (32.7) | 8/16 (50.0) | 1/1 | 3/4 |
| CEPBA double-mut | 0/2 | 0/0 | 0/0 | 35/52 (67.3) | 8/16 (50.0) | 0/1 | 1/4 |
| MLL-PTD | 0/2 | 0/31 | 0/25 | 35/447 (7.8) | 11/241 (4.6) | 3/116 (2.6) | 8/90 (8.9) |
| ASXL1 | 0/0 | 0/39 | 0/4 | 55/447 (12.3) | 62/241 (25.7) | 9/116 (7.8) | 18/90 (20) |
| RUNX1 | 0/0 | 0/2 | 0/11 | 78/447 (17.4) | 59/241 (24.5) | 5/116 (4.3) | 20/90 (22.2) |
| TP53 | 0/33 | 1/40 (2.5) | 0/33 | 5/447 (1.1) | 7/241 (2.9) | 85/116 (73.3) | 17/90 (18.9) |
Within the favorable cytogenetic risk group, cases were not analyzed for all molecular markers because the hierarchical model prognostic assignment was based on the detection of the specific fusion transcript irrespective of further mutations.
TP53 mutation analyses
For molecular analysis of the TP53 gene all 1000 cases were analyzed in exons 4-11 to detect substitutions, insertions, or deletions. Denaturing high-performance liquid chromatography (DHPLC) with subsequent direct Sanger sequencing (n = 190)23 or, alternatively, a sensitive next-generation amplicon deep-sequencing assay was applied (n = 810) using the small-volume Titanium amplicon chemistry assay (Roche Applied Science) as described elsewhere.22 Sample preparation for TP53 deep sequencing was performed in combination with barcode-tagged 48.48 Access Array technology (Fluidigm).34 Next-generation sequencing (NGS) data were analyzed using the GS Variant Analyzer Software 2.5.3 (Roche Applied Science) and Sequence Pilot Version 3.5.2 (JSI Medical Systems). For mutations located in the TP53 gene, PolyPhen2 (http://genetics.bwh.harvard.edu/pph2) was applied using standard parameters to predict the deleterious effects as “probably damaging,” “possibly damaging,” or “benign” of observed single amino acid substitutions on the protein function. A mutation is called “probably damaging” if the probabilistic score is above 0.85, corresponding to a false-positive rate under 10%. For a probabilistic score above 0.15, a mutation is classified as “possibly damaging.” The remaining mutations are classified as “benign.”38 Moreover, the Catalog of Somatic Mutations in Cancer database (COSMIC, http://www.sanger.ac.uk/genetics/CGP/cosmic/) was screened for each mutation to identify aberrations already known as somatic mutations. We additionally screened the International Agency for Research on Cancer (IARC) TP53 database (http://www.p53.iarc.fr/), which is a comprehensive collection of somatic and germline mutations for TP53.
Statistics
Dichotomous variables were compared between different groups using the Fisher exact test and continuous variables by the Student t test. Survival curves were calculated for overall survival (OS) and event-free survival (EFS) according to Kaplan-Meier, and compared using the 2-sided log-rank test. OS was the time from diagnosis of AML to death or last follow-up. EFS was defined as the time from diagnosis of AML to treatment failure, relapse, death, or last follow-up. For all analyses, results were significant at a level of P < .05 at both sides. SPSS Version 14.0.1 software (IBM Corporation) was used for statistical analysis.
Results
Karyotype and its prognostic impact
Based on the karyotype, 1000 patients were assigned to favorable (n = 106), intermediate (n = 688), and unfavorable (n = 206) subgroups according to the refined Medical Research Council (MRC) criteria.3 The prognostic impact of this established cytogenetic classification was confirmed in our cohort (supplemental Figure 2A-B).
Molecular mutations in AML
We detected alterations in NPM1 (282 of 966; 29.2%), RUNX1 (162 of 907; 17.9%), FLT3-ITD (159 of 999; 15.9%), ASXL1 (144 of 937; 15.4%), TP53 (115 of 1000; 11.5%), CEBPA (75 of 997; 7.5%), and MLL-PTD (57 of 952; 6.0%). CEBPA mutations were subdivided into 31 (41.3%) of 75 patients with single mutations and 44 (58.7%) of 75 cases with double mutations. The frequencies of gene mutations in the respective cytogenetic risk groups are given in Table 1. The percentage of patients within the cytogenetic risk groups showing none, 1, 2, or 3 molecular mutations is given in supplemental Table 1.
Mutations in IDH1, IDH2, DNMT3A, and TET2 were analyzed in subsets of patients. IDH1R132 mutations were observed in 56 (7.8%) of 714 patients, IDH2R140 or R172 in 65 (13.7%) of 473 cases, DNMT3A in 56 (27.3%) of 205 patients, and TET2 mutations in 72 (28.8%) of 250 patients, respectively. Details on mutations detected in CEPBA, RUNX1, ASXL1, TET2, and DNMT3A are provided in supplemental Table 2.
Frequency and characterization of TP53 mutations
In 115 patients, a total of 131 TP53 mutations were detected. Ninety-nine patients showed 1, and 16 cases showed 2 TP53 mutations (Figure 1A, supplemental Table 3). The most common mutation types were missense mutations (n = 98; 74.8%), followed by frame-shift (n = 13; 9.9%), splice-site (n = 8; 6.1%), nonsense (n = 7; 5.4%), and in-frame mutations (n = 5; 3.85%). Using the PolyPhen2 software, we identified 85 (86.7%) of 98 missense mutations as probably damaging and 11 (11.2%) of 98 as possibly damaging. Further, 2 mutations were identified as nondamaging, however, both were not present in remission state and were therefore regarded as acquired mutations. TP53 mutations (116 of 131) were included in the COSMIC, and 117 of 131 in the IARC, database. Of those 14 cases which were neither included in the COSMIC nor the IARC database, 11 were frame-shift mutations. The remaining 3 missense mutations were predicted by PolyPhen2 as probably (n = 2) or possibly (n = 1) damaging.
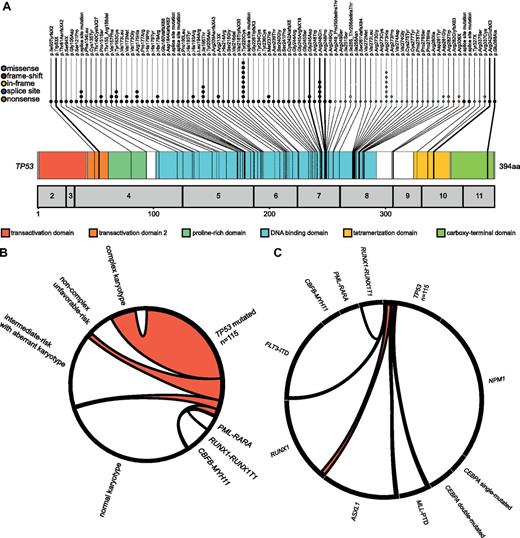
TP53 mutations: localization and association with cytogenetics and other molecular mutations. (A) Molecular mutations in TP53. The numbers of mutations are reflected by the numbers of colored dots which also indicate the mutation effect on the amino acid level. (B) Association between TP53 mutations and karyotype. The Circos plot illustrates the association between TP53 mutations and cytogenetic subgroups. The width of the arches indicates the percentage of positive samples. (C) Mutational complexity of TP53 mutations. The Circos diagram shows the mutational events in patients with TP53 mutations and a respective second molecular mutation. The length of the gene section indicates the number of obtained mutations per marker. The width of the arches indicates the percentage of mutated samples.
TP53 mutations: localization and association with cytogenetics and other molecular mutations. (A) Molecular mutations in TP53. The numbers of mutations are reflected by the numbers of colored dots which also indicate the mutation effect on the amino acid level. (B) Association between TP53 mutations and karyotype. The Circos plot illustrates the association between TP53 mutations and cytogenetic subgroups. The width of the arches indicates the percentage of positive samples. (C) Mutational complexity of TP53 mutations. The Circos diagram shows the mutational events in patients with TP53 mutations and a respective second molecular mutation. The length of the gene section indicates the number of obtained mutations per marker. The width of the arches indicates the percentage of mutated samples.
Overall, the mutations were located predominantly in exon 4 to exon 8 (126 [96.2%] of 131). There was just one mutation in exon 9, three in exon 10, and one mutation detected in exon 11 (Figure 1A). Data on TP53 deletions detected by interphase FISH are provided in supplemental Results and supplemental Figure 3.
Associations between cytogenetics and molecular markers
The frequency of molecular mutations varied between distinct cytogenetic subgroups as depicted in Table 1 and Figure 2 (further details are provided in supplemental Table 4). Within the intermediate-risk group, double-mutated CEBPA, NPM1 mutations, and FLT3-ITD were more frequently observed in cases with normal karyotype (7.8% vs 3.3%, P = .020; 51.0% vs 19.5%, P < .001; 21.3% vs 11.6%, P = .002), whereas ASXL1 and RUNX1 were more frequently mutated within cases with aberrant karyotype (25.7% vs 12.3%, P < .001; 24.5% vs 17.4%, P = .035). The association between TP53 mutations and cytogenetics and other molecular mutations is depicted in Figure 1B and C. Details are provided in supplemental Results. The frequencies of IDH1, IDH2, TET2, and DNMT3A mutations in cytogenetic subgroups are provided in supplemental Table 5.
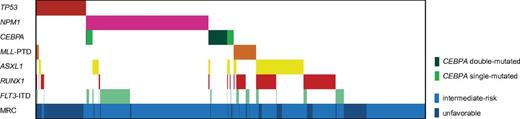
Mutation pattern of AML cases with intermediate and unfavorable cytogenetics. Distributions and frequencies are given for aberrations in TP53, NPM1, CEBPA, MLL-PTD, ASXL1, RUNX1, and FLT3-ITD. The patient cohort is further annotated according to cytogenetics. Cases with favorable risk cytogenetics were not included, as not all molecular markers were analyzed in all cases of this subset because the hierarchical model prognostic assignment was based on the detection of the specific fusion transcript irrespective of further mutations.
Mutation pattern of AML cases with intermediate and unfavorable cytogenetics. Distributions and frequencies are given for aberrations in TP53, NPM1, CEBPA, MLL-PTD, ASXL1, RUNX1, and FLT3-ITD. The patient cohort is further annotated according to cytogenetics. Cases with favorable risk cytogenetics were not included, as not all molecular markers were analyzed in all cases of this subset because the hierarchical model prognostic assignment was based on the detection of the specific fusion transcript irrespective of further mutations.
Associations between molecular markers
Compared with the respective wild-type cases, TP53 was less frequently mutated in cases with the following mutations: CEBPA (0% vs 11.5%, P < .001), NPM1 (0% vs 11.9%, P < .001), FLT3-ITD (0.3% vs 11.2%, P < .001), ASXL1 (0.5% vs 11.7%, P < .001), and RUNX1 (1.0% vs 11.6%, P = .002). The associations between the other molecular markers are depicted in Figure 2 and supplemental Tables 6 and 7. Furthermore, Circos plots depicting the association between each molecular mutation and the other molecular mutations are provided in supplemental Figure 4A through J.
Impact of molecular mutations on outcome
In univariable Cox regression analyses, the following gene mutations were tested: CEBPA single mutations, CEBPA double mutations, NPM1mut/FLT3-ITD−, NPM1mut/FLT3-ITD+, MLL-PTD, RUNX1, ASXL1, and TP53 mutations (Table 2).
Univariable and multivariable Cox regression gene analysis for OS and EFS
| . | Cox regression . | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariable . | Multivariable . | |||||||
| P . | HR . | 95% CI . | P . | HR . | 95% CI . | |||
| Lower . | Upper . | Lower . | Upper . | |||||
| Overall survival | ||||||||
| CEBPA single-mutated | .81 | 0.94 | 0.54 | 1.63 | ||||
| CEBPA double-mutated | .001 | 0.29 | 0.14 | 0.62 | .002 | 0.29 | 0.14 | 0.63 |
| NPM1mut/FLT3-ITD− | < .001 | 0.55 | 0.42 | 0.73 | .002 | 0.62 | 0.46 | 0.84 |
| NPM1mut/FLT3-ITD+ | .48 | 1.14 | 0.8 | 1.63 | ||||
| MLL-PTD | .009 | 1.71 | 1.15 | 2.55 | .117 | 1.39 | 0.92 | 2.08 |
| RUNX1 | .005 | 1.46 | 1.12 | 1.89 | .23 | 1.19 | 0.90 | 1.57 |
| ASXL1 | < .001 | 1.62 | 1.24 | 2.1 | .005 | 1.50 | 1.13 | 1.99 |
| TP53 | < .001 | 3.49 | 2.65 | 4.59 | < .001 | 3.01 | 2.23 | 4.05 |
| Event-free survival | ||||||||
| CEBPA single-mutated | .77 | 0.93 | 0.57 | 1.51 | ||||
| CEBPA double-mutated | .007 | 0.51 | 0.31 | 0.83 | .004 | 0.48 | 0.29 | 0.79 |
| NPM1mut/FLT3-ITD− | .001 | 0.7 | 0.56 | 0.87 | .01 | 0.73 | 0.57 | 0.93 |
| NPM1mut/FLT3-ITD+ | .87 | 0.97 | 0.7 | 1.35 | ||||
| MLL-PTD | < .001 | 1.98 | 1.4 | 2.81 | .006 | 1.65 | 1.12 | 2.36 |
| RUNX1 | .016 | 1.33 | 1.1 | 1.7 | .14 | 1.21 | 0.94 | 1.55 |
| ASXL1 | .064 | 1.26 | 1.0 | 1.6 | ||||
| TP53 | < .001 | 2.96 | 2.27 | 3.85 | < .001 | 2.51 | 1.90 | 3.30 |
| . | Cox regression . | |||||||
|---|---|---|---|---|---|---|---|---|
| Univariable . | Multivariable . | |||||||
| P . | HR . | 95% CI . | P . | HR . | 95% CI . | |||
| Lower . | Upper . | Lower . | Upper . | |||||
| Overall survival | ||||||||
| CEBPA single-mutated | .81 | 0.94 | 0.54 | 1.63 | ||||
| CEBPA double-mutated | .001 | 0.29 | 0.14 | 0.62 | .002 | 0.29 | 0.14 | 0.63 |
| NPM1mut/FLT3-ITD− | < .001 | 0.55 | 0.42 | 0.73 | .002 | 0.62 | 0.46 | 0.84 |
| NPM1mut/FLT3-ITD+ | .48 | 1.14 | 0.8 | 1.63 | ||||
| MLL-PTD | .009 | 1.71 | 1.15 | 2.55 | .117 | 1.39 | 0.92 | 2.08 |
| RUNX1 | .005 | 1.46 | 1.12 | 1.89 | .23 | 1.19 | 0.90 | 1.57 |
| ASXL1 | < .001 | 1.62 | 1.24 | 2.1 | .005 | 1.50 | 1.13 | 1.99 |
| TP53 | < .001 | 3.49 | 2.65 | 4.59 | < .001 | 3.01 | 2.23 | 4.05 |
| Event-free survival | ||||||||
| CEBPA single-mutated | .77 | 0.93 | 0.57 | 1.51 | ||||
| CEBPA double-mutated | .007 | 0.51 | 0.31 | 0.83 | .004 | 0.48 | 0.29 | 0.79 |
| NPM1mut/FLT3-ITD− | .001 | 0.7 | 0.56 | 0.87 | .01 | 0.73 | 0.57 | 0.93 |
| NPM1mut/FLT3-ITD+ | .87 | 0.97 | 0.7 | 1.35 | ||||
| MLL-PTD | < .001 | 1.98 | 1.4 | 2.81 | .006 | 1.65 | 1.12 | 2.36 |
| RUNX1 | .016 | 1.33 | 1.1 | 1.7 | .14 | 1.21 | 0.94 | 1.55 |
| ASXL1 | .064 | 1.26 | 1.0 | 1.6 | ||||
| TP53 | < .001 | 2.96 | 2.27 | 3.85 | < .001 | 2.51 | 1.90 | 3.30 |
Univariable and multivariable Cox regression analysis for OS and EFS of the genes CEBPA (both single- and double-mutated), NPM1mut/FLT3-ITD−, NPM1mut/FLT3-ITD+, MLL-PTD, RUNX1, ASXL1, and TP53.
OS indicates overall survival; EFS, event-free survival; CI, confidence interval; and HR, hazard ratio.
Significantly associated with OS were: CEPBA double mutations (hazard ratio [HR] 0.29; [95% confidence interval (CI), 0.14-0.62]; P = .001), NPM1mut/FLT3-ITD− status (HR 0.55 [0.42-0.73]; P < .001), MLL-PTD (HR 1.71 [1.15-2.55]; P = .009), RUNX1 (HR 1.46 [1.12-1.89]; P = .005), ASXL1 (HR 1.62 [1.24-2.10]; P < .001), and TP53 mutations (HR 3.49 [2.65-4.59]; P < .001).
These significant parameters were entered into a multivariable Cox regression model. The following parameters were independent prognostic factors: CEPBA double mutations (HR 0.29 [0.14-0.63]; P = .002), NPM1mut/FLT3-ITD− status (HR 0.62 [0.46-0.84]; P = .002), ASXL1 (HR 1.50 [1.13-1.99]; P < .005), and TP53 mutations (HR 3.01; [2.23-4.05]; P < .001). The respective data for EFS are depicted in Table 2.
In addition, we performed univariable and multivariable Cox regression analyses including also IDH1, IDH2, DNMT3A, and TET2. None of these genes was significantly associated with OS (for details, see supplemental Table 8 and supplemental Figure 9A-J). Thus, these mutations were not further evaluated.
CEBPA mutations
Of the 75 patients with CEPBA mutations, 31 cases harbored a single CEPBA mutation while 44 cases harbored 2 CEPBA mutations. Patients with double-mutated CEPBA showed longer OS compared with single-mutated cases (OS at 3 years: 78.9% and 38.5%, P = .014). Differences in EFS did not reach significance (EFS after 3 years: 53.9% and 36.6%, P = .108). OS and EFS of CEBPA single-mutated cases were comparable with CEPBA wild-type cases (OS at 3 years: 38.5% and 43.6%, P = .689, EFS at 3 years: 36.6% and 29.4%, P = .678). The Kaplan-Meier plots for OS and EFS are depicted in supplemental Figure 5A through H. OS and EFS of CEBPA double-mutated cases are comparable with patients with AML with t(15;17)(q22;q12)/PML-RARA (OS after 3 years: 78.9% and 86.1%, P = .597, EFS at 3 years: 53.9% and 82.5%, P = .031; supplemental Figure 5I-J).
NPM1 mutations
In our cohort, we confirmed that in AML with normal karyotype, patients with NPM1mut/FLT3-ITD− (n = 152) had a better outcome compared with patients with NPM1mut/FLT3-ITD+ (n = 53; OS at 3 years 66.8% vs 43.0%, P = .008; EFS at 3 years 44.0% vs 34.5%, P = .171, supplemental Figure 6A-B). In addition, in the complete intermediate-risk group outcome in patients with NPM1mut/FLT3-ITD− (n = 182) was also superior compared with patients with NPM1mut/FLT3-ITD+ (n = 62) as depicted in supplemental Figure 6C-D (OS at 3 years, 63.8% vs 41.6%, P = .005; EFS at 3 years, 41.5% vs 34.2%, P = .172).
MLL-PTD, RUNX1, and ASXL1 mutations
In univariable Cox regression analysis, MLL-PTD, RUNX1, and ASXL1 mutations were all associated with shorter OS and EFS. As combinations of these 3 mutations frequently occur (supplemental Figure 7A), patients with at least 1 mutation in 1 of these 3 genes were grouped together. Patients with MLL-PTD and/or RUNX1 and/or ASXL1 mutations (n = 228; ASXL1 sole, n = 71; RUNX1 sole, n = 69; MLL-PTD sole, n = 25; ASXL1 and RUNX1, n = 42; ASXL1 and MLL-PTD, n = 2; RUNX1 and MLL-PTD, n = 19; ASXL1 and RUNX1 and MLL-PTD, n = 2) showed shorter OS and EFS than patients without MLL-PTD, RUNX1, and ASXL1 mutations (n = 583): OS at 3 years, 22.5% vs 50.3%, P < .001; EFS at 3 years, 11.1% vs 34.0%, P < .001, respectively (supplemental Figure 7B-D).
TP53 mutations
In univariable and multivariable Cox regression analyses, TP53 mutations were associated with the shortest survival. Median OS in patients with TP53 mutation (n = 80) was 4.6 months compared with 35.6 months in TP53 wild-type cases (n = 761; P < .001), data for median EFS was 3.1 compared with 13.3 months (P < .001); OS and EFS at 3 years was 0% vs 49.6% and 0% vs 33.8%, respectively. Interestingly, even within the complex karyotype cohort, patients with TP53 mutations (n = 56/80) showed an inferior outcome than TP53 wild-type cases (n = 24/80; OS and EFS at 3 years: 0% vs 27.9%, P = .002, 0% vs 25.7%, P = .002; supplemental Figure 8A-F). Data on the prognostic impact of TP53 mutations in relation to TP53 deletions is provided in supplemental Figure 8G and H.
Prognostic model based on molecular markers only
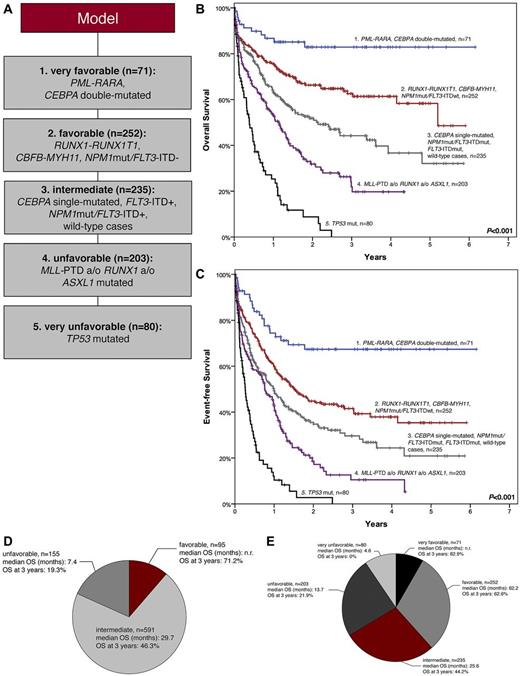
A prognostic model using molecular markers is proposed as follows (Figure 3A). The cohort was stratified according to a primarily “entity-based” hierarchy taking the following into account: (1) presence of either PML-RARA (n = 29), CBFB-MYH11 (n = 31), or RUNX1-RUNX1T1 (n = 35); (2) CEBPA double-mutated status (n = 42); (3) NPM1 mutations (n = 250), with patients in this cohort further subdivided according to their FLT3-ITD status (NPM1mut/FLT3-ITD−, n = 186; NPM1mut/FLT3-ITD+, n = 64). Of the remaining patients, those with TP53 mutations (n = 80) were separated. TP53 wild-type cases were assigned to the molecular unfavorable group if they showed at least 1 of the following mutations: MLL-PTD, ASXL1mut, RUNX1mut (n = 203). Patients showing none of these mutations were assigned to the intermediate group (n = 171).
Model based on molecular mutations. (A) Categorization of all mutations detected in AML to 5 prognostic groups. The cohort was stratified according to an “entity-based” hierarchy: (1) PML-RARA, CEBPA double-mutated (n = 71); (2) RUNX1-RUNX1T1, CBFB-MYH11, NPM1mut/FLT3-ITD− (n = 252); (3) CEBPA single-mutated, NPM1mut/FLT3-ITD+, FLT3-ITD+, wild-type cases (n = 235); (4) MLL-PTD and/or RUNX1mut and/or ASXL1mut (n = 203); (5) TP53 mutations (n = 80). (B-C) Kaplan-Meier plots for the molecular mutation-based model, which separates patients into 5 prognostic subgroups: (1) PML-RARA, CEBPA double-mutated (n = 71); (2) RUNX1-RUNX1T1, CBFB-MYH11, NPM1mut/FLT3-ITD− (n = 252); (3) CEBPA single-mutated NPM1mut/FLT3-ITD+, FLT3-ITD+, wild-type cases (n = 235); (4) MLL-PTD and/or RUNX1 and/or ASXL1 (n = 203); (5) TP53 mutations (n = 80). (B) OS (P values between the subgroups are 1 vs 2, P = .004; 2 vs 3, P = .001; 3 vs 4, P = .001; 4 vs 5, P < .001, respectively). (C) EFS (P values between the subgroups are 1 vs 2, P = .001; 2 vs 3, P = .011; 3 vs 4, P = .004; 4 vs 5, P < .001, respectively). (D-E) Comparison of cytogentic and molecular genetic model. Cohort subdivided according to (D) cytogenetics and (E) molecular mutations. The model based on molecular mutations leads to 5 prognostic subsets instead of 3 in the cytogenetic model and thus a refined assignment of patients to distinct prognostic groups with large differences in outcome.
Model based on molecular mutations. (A) Categorization of all mutations detected in AML to 5 prognostic groups. The cohort was stratified according to an “entity-based” hierarchy: (1) PML-RARA, CEBPA double-mutated (n = 71); (2) RUNX1-RUNX1T1, CBFB-MYH11, NPM1mut/FLT3-ITD− (n = 252); (3) CEBPA single-mutated, NPM1mut/FLT3-ITD+, FLT3-ITD+, wild-type cases (n = 235); (4) MLL-PTD and/or RUNX1mut and/or ASXL1mut (n = 203); (5) TP53 mutations (n = 80). (B-C) Kaplan-Meier plots for the molecular mutation-based model, which separates patients into 5 prognostic subgroups: (1) PML-RARA, CEBPA double-mutated (n = 71); (2) RUNX1-RUNX1T1, CBFB-MYH11, NPM1mut/FLT3-ITD− (n = 252); (3) CEBPA single-mutated NPM1mut/FLT3-ITD+, FLT3-ITD+, wild-type cases (n = 235); (4) MLL-PTD and/or RUNX1 and/or ASXL1 (n = 203); (5) TP53 mutations (n = 80). (B) OS (P values between the subgroups are 1 vs 2, P = .004; 2 vs 3, P = .001; 3 vs 4, P = .001; 4 vs 5, P < .001, respectively). (C) EFS (P values between the subgroups are 1 vs 2, P = .001; 2 vs 3, P = .011; 3 vs 4, P = .004; 4 vs 5, P < .001, respectively). (D-E) Comparison of cytogentic and molecular genetic model. Cohort subdivided according to (D) cytogenetics and (E) molecular mutations. The model based on molecular mutations leads to 5 prognostic subsets instead of 3 in the cytogenetic model and thus a refined assignment of patients to distinct prognostic groups with large differences in outcome.
This classification allowed the separation of 5 prognostic subgroups with large differences in outcome (Figure 3A): (1) PML-RARA, CEBPA double-mutated (n = 71); (2) RUNX1-RUNX1T1, CBFB-MYH11, NPM1mut/FLT3-ITD− (n = 252); (3) CEBPA single-mutated, NPM1mut/FLT3-ITD+, FLT3-ITD+, wild-type cases (n = 235); (4) MLL-PTD and/or RUNX1mut and/or ASXL1mut (n = 203); (5) TP53 mutations (n = 80): median OS was not reached (n.r.) vs 62.2 vs 25.6 vs 13.7 vs 4.6 months, respectively, OS at 3 years was 82.9% vs 62.6% vs 44.2% vs 21.9% vs 0%, respectively, P < .001 (Figure 3B); median EFS was n.r. vs 18.1 vs 11.8 vs 9.1 vs 3.1 months, respectively, EFS at 3 years was 67.4% vs 41.6% vs 29.7% vs 10.5% vs 0%, respectively, P < .001 (Figure 3C). This compares favorably to the cytogenetic model which provides only 3 prognostic risk groups according to the refined MRC classification3 as follows: median OS n.r. vs 29.7 vs 7.4 months, respectively, and OS at 3 years 71.2% vs 46.3% vs 19.3%, respectively (P < .001; supplemental Figure 2A-B). The distribution of patients within the 2 models is depicted in Figure 3D and E.
This molecular prognostic model was further evaluated separately for adults 18 to 60 years of age and for those older than 60 years, and showed prognostic impact in both age groups (supplemental Figure 10A-D, supplemental Table 9). The proportions of the 5 novel prognostic subgroups vary between the different age subgroups; details are provided in supplemental Figure 11. A multivariable Cox regression analysis including age as a continuous variable and the novel molecular score revealed an independent impact of both parameters on OS (for both P < .0001, relative risk for age was 1.45 per 10 years, and for the novel model was 1.48 per step).
In addition, we provided an alternative model based on molecular markers only, which assigned patients according to the worst molecular parameter detected. Details are provided in supplemental Figure 12A through C.
Comparison of the new molecular model with previously published models
To compare our proposed model with published models which include cytogenetic and molecular genetic markers, we analyzed our cohort according to the prognostic model proposed by ELN Working Group18 and the model proposed by Patel and colleagues.39 Detailed data are provided in supplemental Table 10 and supplemental Figures 14A and B and 15A and B. In summary, both previously published models showed significant impact on OS and EFS in our cohort. However, our new molecular model allows the separation into 5 prognostic categories comprising 8.5%, 30.0%, 27.9%, 24.1%, and 9.5%, respectively, while the ELN model separates into 4 groups comprising 18.0%, 39.7%, 23.0%, and 19.3%, respectively. A subset of 596 (70.9%) of 841 cases of our cohort were categorized according to the model proposed by Patel et al with 16.9% assigned to the favorable group, 14.5% to the intermediate group, and 39.5% to the unfavorable group, respectively. More importantly, the OS at 3 years varies over a broader range from 82.9% to 0% in our model compared with 64.1% to 21.5% in the ELN model, and 69.2% to 27.4% according to the model by Patel et al. Thus, our model identifies on one hand 8.5% of patients who have an excellent prognosis of 82.9% OS at 3 years with conventional treatment, while the good-risk groups of the ELN and Patel model show a 3-year-OS of only 64.1% and 69.2%, respectively. Thus, our model allows the identification of a subset of patients with excellent prognosis. This subgroup might qualify for a reduction of treatment intensity. Furthermore, our model defines a subgroup of patients with a very unfavorable outcome (1-year survival: 25.3%; 3-year survival: 0%). This subset of patients might qualify for upfront selection for experimental approaches or allogeneic stem cell transplantation.
Discussion
In AML, the impact of genetic characterization on estimation of prognosis and treatment stratification has increased over the past years. The molecular characterization will become even more important in the future as the development of novel targeted therapy approaches such as FLT3-pathway inhibitors rely on distinct biomarkers. In addition, several molecular markers can be used for MRD detection. Consequent monitoring of response to therapy will allow an individually tailored treatment in AML.30,40-44 So far, assessment of prognosis at diagnosis was based on the karyotype3 and predominantly in the subset of cases with normal karyotype on molecular mutations.6-13,29
To improve the prognostic model based on cytogenetics, established molecular markers and—for the first time—TP53 mutation analysis were used for prognostication in all cytogenetic subgroup AML. This approach allowed for the characterization of 5 prognostic subsets with significant differences in OS. Furthermore, replacing the cytogenetic detection of favorable subgroups [t(15;17), t(8;21) inv(16)/t(16;16)] by RT-PCR–based methods and mutation screening of 7 genes (duplications within the FLT3 and MLL genes and mutations in NPM1, ASXL1, RUNX1, CEPBA, and TP53) led to a model based on molecular markers only and thus enabled a promising novel diagnostic approach not relying on labor-intensive and time-consuming cytogenetics. For the first time, these molecular markers were evaluated in combination in such a large dataset.
An impact on outcome in AML has been described for further genes such as DNMT3A, IDH1/2, and TET2. However, published data on the prognostic impact of these mutations is conflicting. The study by Ley et al who described for the first time the occurrence of DNMT3A mutations in AML observed a significantly shorter OS in patients with DNMT3A mutations to those without in the total AML cohort (n = 218) as well as in patients with intermediate-risk karyotype.16 However, in a study by Gaidzik et al comprising 1218 AML patients, no clear impact of DNMT3A mutations was observed in the total cohort, while a negative impact on OS was observed in the subset of AML with NPM1 wild type.45 Furthermore, for IDH1 and IDH2 mutations, no clear impact on OS was observed in 2 large studies.36,46,47 With respect to TET2 mutations, an impact on OS was observed only in small subsets of patients.37,48,49 To test the impact of these recently identified markers in our cohort, we analyzed a subset of patients for mutations within these genes. Neither DNMT3A, IDH1, IDH2 nor TET2 were found to significantly impact on OS in contrast to the molecular abnormalities finally included in the novel prognostic model. As for TET2 mutations, a trend toward a negative impact on OS was observed; we tested whether TET2 mutations influenced outcome in one of the newly defined prognostic subgroups. However, in none of the subgroups was a significant impact observed, thus TET2 mutations were not included in the model. The prognostic relevance of the single markers finally included into our new molecular-based model has been demonstrated by us and others in previous studies. Several studies have been published establishing the prognostic impact of PML-RARA, RUNX1-RUNX1T1, CBFB-MYH11, mutations in NPM1,9 RUNX1,10 CEPBA, FLT3-ITD,8 and MLL-PTD.29 The negative prognostic impact of ASXL1 mutations in AML has only recently been demonstrated.11,33,50 However, these studies included up to over 1000 patients suggesting high reliability of these analyses. For TP53 mutations, data on prognosis in AML is rare; however, an association of TP53 mutations and complex karyotype has been reported suggesting a negative impact on OS,23,24 which was clearly proven in the presented study. Thus, strikingly, even with the inclusion of only 10 molecular markers, the novel prognostic model outperforms the currently used cytogenetic prognostic models as it separates the total cohort of AML into 5 distinct prognostic subgroups with significant differences in OS.
The first step of the development of the novel prognostic model was to evaluate the prognostic impact of single markers. In line with published data, we confirmed that double-mutated CEBPA was independently associated with favorable outcome while single mutations were not.13,51 OS of patients with double-mutated CEBPA was comparable with patients with PML-RARA rearrangement. NPM1 mutations have been established as a favorable prognostic marker in AML with normal karyotype. In NPM1mut cases, the negative prognostic impact of concomitant FLT3-ITD was shown, thus the genotype NPM1mut/FLT3-ITD− is associated with favorable outcome while NPM1mut/FLT3-ITD+ is not. This was also the case in our cohort. The OS of patients with NPM1mut/FLT3-ITD− was comparable with patients with CBF leukemias (62.6% and 63.3% at 3 years). Thus, both were combined into the favorable molecular subset.
The negative prognostic impact of RUNX1 mutations was published in subsets of AML as well as in the total AML cohort.6,10 ASXL1 mutations were recently identified in AML and a negative impact on survival was demonstrated.11,33,50 MLL-PTD occur less frequently than the mutations mentioned before, but the negative prognostic impact was confirmed in several studies.29,52,53 In the presented study, the negative prognostic impact of MLL-PTD, RUNX1 mutations, or ASXL1 mutations was confirmed. These 3 molecular alterations frequently occur together. They were grouped together into the unfavorable subset with an OS at 3 years of 21.9%.
The previously mentioned molecular mutations showed the highest frequency within the intermediate-risk cytogenetic group. TP53 alterations were a promising molecular marker to be associated with unfavorable cytogenetics. This prompted us to study the frequency and prognostic impact in AML. The highest frequency of TP53 mutations was observed in AML with unfavorable cytoge-netics (49.5%) and particularly so in AML with complex karyotype (73.3%). This high frequency in AML with complex karyotype was comparable with published data.23,24 In addition, we demonstrated that TP53 mutations are very rare in other cytogenetic subgroups. Based on cytogenetics, the unfavorable risk group comprises 18% of all AML cases with 19.3% surviving 3 years. This is comparable with 21.9% OS observed for the newly defined unfavorable molecular risk group comprising 24% of patients. However, TP53 mutations were observed in 9.5% of AML patients with an OS at 3 years of 0%. Thus, adding TP53 mutation analysis to the characterization of AML allows the separation of the subset with the most unfavorable outcome. In comparison to the cytogenetic model proposed by MRC and the models proposed by the ELN and Patel et al relying on cytogenetics and molecular markers, our new model allows a better prognostic separation as OS at 3 years varies over a broader range from 82.9% to 0% in our model compared with 71.2% to 19.3% in the MRC cytogenetic model,3 64.1% to 21.5% in the ELN model,18 and 69.2% to 27.4% according to the model by Patel et al.39 Thus, our model identifies on one hand patients who have an excellent prognosis with conventional treatment and on the other hand allows the selection of 9.5% of patients with an extremely low chance for cure with conventional treatment who might benefit from upfront experimental approaches or upfront allogeneic stem cell transplantation.
Using novel sequencing technologies, a model based on molecular markers only allows a rapid single-step risk assessment as a basis for treatment decisions. In contrast to the recently published study by Patel et al who developed a prognostic model for AML based on cytogenetics and 18 molecular markers in younger patients (< 60 years),39 we here propose a model with prognostic impact for younger and elderly AML patients which requires 10 molecular markers (TP53, ASXL1, RUNX1, CEPBA, NPM1, FLT3-ITD, MLL-PTD, PML-RARA, CBFB-MYH11, RUNX1-RUNX1T1) and no additional cytogenetic investigation. To directly compare our new model relying on molecular markers only with other currently proposed models, we additionally evaluated our cohort according to the following models: the MRC-refined model based on cytogenetics only,3 the ELN model18 using both cytogenetics and 3 molecular markers, and the model proposed by Patel and colleagues39 relying on cytogenetics and 18 molecular markers, respectively. All 3 previously published models showed significant prognostic impact in our cohort. However, our model had several advantages compared with them as it allows the separation into 5 distinct prognostic groups compared with 3 and 4 groups, respectively. As such, prognosis was more distinctly determined and the differences in OS between the prognostic subgroups were overall larger than in the published models. The approach to substitute chromosome banding analysis by molecular techniques meets the challenges raised by Godley switching the future evaluation of AML from conventional methods toward novel technologies such as NGS with rapid results to even choose induction treatment based on this molecular genetic characterization.54 Furthermore, such a comprehensive molecular characterization at diagnosis provides a molecular marker in the majority of cases (73.5%), which could be used as targets for MRD monitoring and individualization of therapy. The clinical impact of MRD monitoring in AML based on quantitative RT-PCR has recently been reviewed.40 In addition, first data on the applicability of MRD detection with high sensitivity on NGS platforms is available.55,56 However, the general feasibility and the clinical usefulness of MRD monitoring using novel markers has to be demonstrated and proven in future prospective studies.
In conclusion, molecular screening for the recurrent balanced rearrangements (PML-RARA, RUNX1-RUNX1T1, CBFB-MYH11) and mutation analyses in CEBPA, NPM1, RUNX1, ASXL1, and TP53 as well as for FLT3-ITD and MLL-PTD leads to a novel prognostic classification in AML that improves any prognostic model based on cytogenetics and is easy to be investigated and applied. Five instead of 3 risk groups can be identified with large differences in outcome. OS in the very favorable molecular risk group at 3 years is above 80%. An individualized therapy and possibly reduction of therapy intensity based on monitoring of MRD is an option. While 19.3% of patients with unfavorable cytogenetics survive 3 years, none of the patients with TP53 mutation did. Thus, for the latter group, novel treatment approaches ranging between palliative care and new targeted therapy protocols need to be discussed. The molecularly based subdivision of the large intermediate-risk cytogenetic group may prove helpful to analyze the effect of novel therapeutic options in more detail, especially in combination with MRD monitoring enabled by the individual molecular markers.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank L. Böck, M. Staller, and T. Alpermann for excellent assistance, and all coworkers at the MLL Munich Leukemia Laboratory for approaching together many aspects in the field of leukemia diagnostics and research by their dedicated work. The authors thank all physicians for providing and caring for patients as well as collecting data. Centers and investigators (contributing 10 or more) are listed in order of number of cases provided: St Johannes Hospital Dortmund (V. Hagen), Klinikum Osnabrück (R. Peceny), Asklepios Klinik St Georg, Hamburg (N. Schmitz), Johanniter-Krankenhaus Bonn (Y. Ko), Asklepios Klinik Altona Hamburg (D. Braumann), Klinikum Fulda (H.-G. Höffkes), Klinikum Lippe-Lemgo (F. Hartmann), Städtisches Klinikum Kassel (M. Wolf), Franziskus Hospital Bielefeld (H.-J. Weh), Klinikum Krefeld (M. Planker), Klinikum Rechts der Isar der Technischen Universität München (K. Götze), Klinikum Rosenheim (G. Puchtler), Städtisches Krankenhaus Gütersloh (G. Massenkeil), Evangelisches Krankenhaus Hamm (E. Lange), Klinikum Bremen-Mitte (B. Hertenstein), Krankenhaus Düren (M. Flaßhove), Kreiskrankenhaus Gummersbach (M. Sieber), St Marien-Krankenhaus Siegen (W. Gassmann).
Authorship
Contribution: V.G. and C.H. designed the study, interpreted the data, and wrote the manuscript; V.G., S.S., C.E., and F.D. performed molecular analysis; S.S., A.K., W.K., and T.H. provided assistance in the design of the study, characterized patient samples, and critically reviewed the manuscript; A.R. performed bioinformatics; C.S., C.-M.W., P.S., H.S., and K.-A.K. provided samples and clinical data, contributed to the interpretation of the data, and critically reviewed the draft; and all authors approved the final version submitted for publication.
Conflict-of-interest disclosure: C.H., S.S., W.K., and T.H. have equity ownership of MLL Munich Leukemia Laboratory GmbH. V.G., A.K. C.E., F.D., and A.R. are employed by MLL Munich Leukemia Laboratory GmbH. The remaining authors declare no competing financial interests.
Correspondence: Claudia Haferlach, MD, MLL Munich Leukemia Laboratory, Max-Lebsche-Platz 31, 81377 Munich, Germany; e-mail: claudia.haferlach@mll.com.