Abstract
Subclones homozygous for JAK2V617F are more common in polycythemia vera (PV) than essential thrombocythemia (ET), but their prevalence and significance remain unclear. The JAK2 mutation status of 6495 BFU-E, grown in low erythropoietin conditions, was determined in 77 patients with PV or ET. Homozygous-mutant colonies were common in patients with JAK2V617F-positive PV and were surprisingly prevalent in JAK2V617F-positive ET and JAK2 exon 12-mutated PV. Using microsatellite PCR to map loss-of-heterozygosity breakpoints within individual colonies, we demonstrate that recurrent acquisition of JAK2V617F homozygosity occurs frequently in both PV and ET. PV was distinguished from ET by expansion of a dominant homozygous subclone, the selective advantage of which is likely to reflect additional genetic or epigenetic lesions. Our results suggest a model in which development of a dominant JAK2V617F-homzygous subclone drives erythrocytosis in many PV patients, with alternative mechanisms operating in those with small or undetectable homozygous-mutant clones.
Introduction
The JAK2V617F mutation is found in ∼ 95% of patients with polycythemia vera (PV) and 60% of those with essential thrombocythemia (ET),1-4 but the mechanisms responsible for the different disease phenotypes remain unclear. Several lines of circumstantial evidence suggest that increased signaling through mutant JAK2 is important: (1) A “homozygous” sequence pattern in granulocytes was identified in ∼ 30% of patients with PV but was rare in ET1-4 ; (2) in PV patients, JAK2V617F allele burden correlates with higher hemoglobin levels and white cell counts but lower platelet counts5,6 ; (3) the copy number of mutant JAK2 influences phenotype in mouse models7,8 ; and (4) JAK2 exon 12 mutations are reported to signal more strongly than JAK2V617F and are associated with PV but not ET.9
Homozygosity for JAK2V617F results from mitotic recombination,1-4 and homozygous-mutant BFU-E were present in most patients with PV but not in those with ET.10 This observation raised the possibilities that patients with PV are more prone to develop a homozygous subclone or that homozygous-mutant cells have a selective advantage in patients with PV but not in those with ET. In either case, it has been widely assumed that homozygous-mutant cells in a given patient are usually members of a single clone with a selective advantage and that JAK2V617F homozygosity plays a causal role in PV pathogenesis. However, this model is complicated by several observations: some patients with PV have very small homozygous-mutant clones10 ; 2 individual patients have been reported to harbor 2 distinct homozygous clones11,12 ; a defect in STAT1 phosphorylation has been identified in PV patients13 ; and reports of small numbers of ET patients who harbor homozygous-mutant BFU-E14-16 and of PV patients with none.14,15 We have therefore systematically assessed the prevalence and clonal relationship of homozygous-mutant BFU-E precursors in patients with JAK2-mutated PV and ET. In contrast to our previous study,10 we used low erythropoietin conditions and analyzed a larger number of colonies, to maximize identification of mutant precursors.
Methods
Patient selection
For patient recruitment sites, see supplemental Methods (available on the Blood Web site; see the Supplemental Materials link at the top of the online article). The study was approved by the Cambridge and Eastern Region Ethics Committee, patients gave written informed consent, and research was carried out in accordance with the Declaration of Helsinki. Patients met British Committee for Standards in Haematology diagnostic criteria for PV or ET.17,18 Clinical features are shown in supplemental Table 1.
BFU-E assays
PBMCs were plated (2.2-2.5 × 105 cells/mL) in Methocult H4035 (StemCell Technologies), supplemented with 0.01 or 0.1 U/mL recombinant erythropoietin-alfa (Janssen-Cilag) and incubated at 37°C for 14-16 days. BFU-E colonies were identified by morphology and/or cytospins.
Mutation screening
Colony DNA was prepared by isopropanol extraction from RLT lysis buffer (QIAGEN), or suspension in water. Genotyping for JAK2V617F was performed by quantitative PCR19 or direct sequencing and for exon 12 mutations by direct sequencing. For TET2 analysis, coding regions and splice sites were sequenced using granulocyte DNA. Primers are listed in supplemental Table 2.
Microsatellite analysis
Fluorescence microsatellite PCR used primer sequences from public databases (supplemental Table 2) and DNA from buccal swabs or colonies. Analysis was performed using a 3730xl DNA analyser and Peak Scanner Version 1.0 software (Applied Biosytems).
Results and discussion
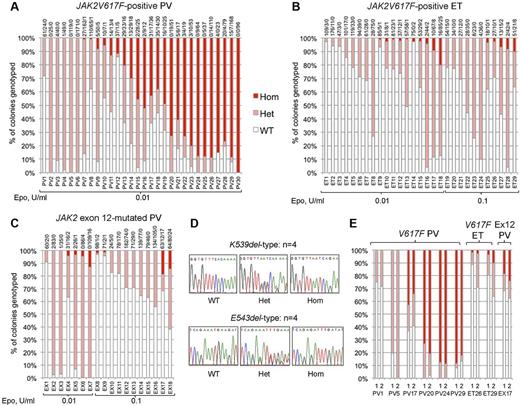
We genotyped a total of 6495 BFU-E colonies from 77 patients: 30 with JAK2V617F-positive PV, 29 with JAK2V617F-positive ET, and 18 with JAK2 exon 12-mutated PV. Homozygous-mutant precursors were present in all 3 disease groups (Figure 1A-C). Patients with JAK2V617F-positive PV (Figure 1A) showed the highest proportions of homozygous-mutant precursors, consistent with previous reports.10,14,15,20 Homozygosity was undetectable in 6 patients with JAK2V617F-positive PV (20%), despite assessment of a large number of colonies and the use of low erythropoietin conditions that select for JAK2V617F-homozygous precursors. Acquired TET2 mutations were reported in 3 of 5 such “heterozygous-only” patients14,21 but were not found in our 6 patients, indicating that many such patients do not carry TET2 mutations.
Proportions of JAK2 genotypes in BFU-Es from patients with JAK2-mutated PV and ET. Each vertical bar represents 1 patient, divided according to the proportion of wild-type, heterozygous, and homozygous-mutant colonies obtained, with the absolute colony numbers shown above (WT/Het/Hom). BFU-E colonies were grown under low erythropoietin conditions as indicated. (A) Colony genotypes for 30 patients with JAK2V617F-positive PV (total 2287 colonies; mean 76 colonies per patient). (B) Colony genotypes for 29 patients with JAK2V617F-positive ET (total 2277 colonies; mean 79 per patient). (C) Colony genotypes for 18 patients with JAK2 exon 12-mutated PV (total 1931 colonies; mean 107 per patient). (D) Example sequence traces for patients with patients with homozygous JAK2 exon 12 mutations in colonies. (E) Examples of patients grown on 2 occasions show reproducibility of genotype proportions in JAK2V617F-positive PV, JAK2V617F-positive ET, and JAK2 exon 12-mutated PV (1 and 2 represent independent experiments). In total, 16 patients (5 “heterozygous-only” JAK2V617F-positive PV patients, 4 JAK2V617F-positive PV patients with homozygous and heterozygous clones, 3 JAK2V617F-positive ET patients with small homozygous clones, and 4 JAK2 exon 12-mutated PV patients with homozygous clones) were assessed in this way (mean time between experiments, 13 months; range, 2-32 months) and showed reproducibility of proportions of heterozygous and homozygous-mutant colonies.
Proportions of JAK2 genotypes in BFU-Es from patients with JAK2-mutated PV and ET. Each vertical bar represents 1 patient, divided according to the proportion of wild-type, heterozygous, and homozygous-mutant colonies obtained, with the absolute colony numbers shown above (WT/Het/Hom). BFU-E colonies were grown under low erythropoietin conditions as indicated. (A) Colony genotypes for 30 patients with JAK2V617F-positive PV (total 2287 colonies; mean 76 colonies per patient). (B) Colony genotypes for 29 patients with JAK2V617F-positive ET (total 2277 colonies; mean 79 per patient). (C) Colony genotypes for 18 patients with JAK2 exon 12-mutated PV (total 1931 colonies; mean 107 per patient). (D) Example sequence traces for patients with patients with homozygous JAK2 exon 12 mutations in colonies. (E) Examples of patients grown on 2 occasions show reproducibility of genotype proportions in JAK2V617F-positive PV, JAK2V617F-positive ET, and JAK2 exon 12-mutated PV (1 and 2 represent independent experiments). In total, 16 patients (5 “heterozygous-only” JAK2V617F-positive PV patients, 4 JAK2V617F-positive PV patients with homozygous and heterozygous clones, 3 JAK2V617F-positive ET patients with small homozygous clones, and 4 JAK2 exon 12-mutated PV patients with homozygous clones) were assessed in this way (mean time between experiments, 13 months; range, 2-32 months) and showed reproducibility of proportions of heterozygous and homozygous-mutant colonies.
In JAK2V617F-positive ET and JAK2 exon 12-mutated PV, JAK2V617F-homozygous colonies were identified in a surprisingly large percentage of patients (52% and 44%, respectively). Homozygous clone sizes were small; and in exon 12-mutated PV, homozygosity was associated with both K539L-type and E543del-type mutations (Figure 1D). The relative proportions of heterozygous and homozygous-mutant colonies were stable over time in 16 patients tested on 2 separate occasions (Figure 1E; and data not shown). Homozygous-mutant BFU-E are therefore a persistent feature in many patients with JAK2V617F-positive PV, JAK2V617F-positive ET, and JAK2 exon 12-mutated PV, and are more frequent than previously recognized in the latter 2 disorders.5,20,22,23 Comparison of patients with and without detectable homozygosity did not reveal any differences in blood counts at diagnosis, presence of palpable splenomegaly, or thrombotic history (Mann-Whitney U test/Fisher exact tests, P > .05 for each disease subgroup).
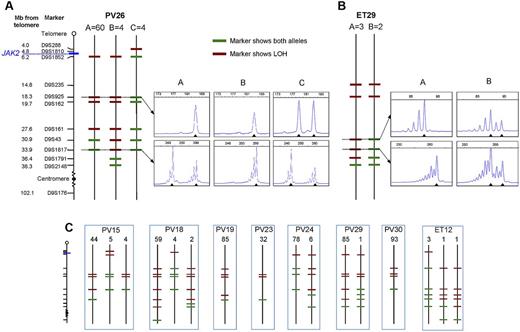
To determine whether JAK2V617F-homozygous colonies were part of a single clone or reflected recurrent acquisition of loss of heterozygosity (LOH), breakpoints for chromosome 9p LOH were mapped using fluorescence microsatellite PCR in 576 homozygous-mutant colonies from 10 patients (8 PV and 2 ET). Results for 1 PV patient and 1 ET patient are shown in Figure 2A and B, with the others summarized in Figure 2C. At least 2 distinct homozygous subclones were identified in 5 of 8 PV patients and both ET patients, indicating that independent homozygous-mutant clones arise frequently in both PV and ET. Importantly, the resolution of breakpoint mapping was limited (2.3-14.2 MB), so the number of distinct subclones may be an underestimate. The high prevalence of homozygous-mutant clones may reflect a role for JAK2V617F in homologous recombination24 and/or inappropriate survival of cells after DNA damage.25 There was no obvious relationship between the presence of multiple homozygous-mutant subclones and patient age, disease duration, or therapy (supplemental Table 3). Homozygous-mutant colonies did not arise from short-lived progenitors because distinct subclones persist over time. Patient PV24 had 2 subclones whose relative proportions remained unchanged over 10 months (supplemental Figure 1), indicating that they arose from early stem/progenitor cells.
Microsatellite mapping of 9p LOH in JAK2V617F-homozygous colonies. (A) Microsatellite mapping of 68 JAK2V617F-homozygous colonies from patient PV26 (patient codes are the same as in Figure 1). The panel of microsatellite markers on chromosome 9 is shown on the left (distances are to scale between the telomere and D9S2148), from which informative markers were selected for each patient. Three patterns of microsatellite markers were observed in colonies from this patient, indicating 3 distinct LOH breakpoints. Each subclone is represented by a vertical line denoted A (60 colonies), B (4 colonies), or C (4 colonies), with markers showing heterozygosity in green and those showing LOH in red. Example traces from fluorescence microsatellite PCR are shown for 2 markers (D9S925 and D9S1817) for the 3 subclones (right), with the position of alleles shown by arrowheads. (B) Microsatellite mapping of 5 JAK2V617F-homozygous colonies from patient ET29. Two patterns of microsatellite markers were observed; example traces are shown for 2 markers (D9S43 and D9S1817) on the right. (C) Microsatellite mapping of JAK2V617F-homozygous colonies in 8 other patients. The panel of markers is the same as in panel A. Numbers of colonies corresponding to each pattern are shown above.
Microsatellite mapping of 9p LOH in JAK2V617F-homozygous colonies. (A) Microsatellite mapping of 68 JAK2V617F-homozygous colonies from patient PV26 (patient codes are the same as in Figure 1). The panel of microsatellite markers on chromosome 9 is shown on the left (distances are to scale between the telomere and D9S2148), from which informative markers were selected for each patient. Three patterns of microsatellite markers were observed in colonies from this patient, indicating 3 distinct LOH breakpoints. Each subclone is represented by a vertical line denoted A (60 colonies), B (4 colonies), or C (4 colonies), with markers showing heterozygosity in green and those showing LOH in red. Example traces from fluorescence microsatellite PCR are shown for 2 markers (D9S925 and D9S1817) for the 3 subclones (right), with the position of alleles shown by arrowheads. (B) Microsatellite mapping of 5 JAK2V617F-homozygous colonies from patient ET29. Two patterns of microsatellite markers were observed; example traces are shown for 2 markers (D9S43 and D9S1817) on the right. (C) Microsatellite mapping of JAK2V617F-homozygous colonies in 8 other patients. The panel of markers is the same as in panel A. Numbers of colonies corresponding to each pattern are shown above.
Importantly, patients with PV and ET differed in that the former harbored a major homozygous-mutant clone that was 8-85 times the size of minor subclones in the same patient (Figure 2A-C). This observation demonstrates that the large numbers of homozygous-mutant colonies present in most PV patients do not reflect accumulation of numerous independent subclones but rather the expansion of 1 dominant clone. Given the circumstantial evidence that JAK2V617F homozygosity enhances erythropoiesis, it seems probable that the dominant clone present in many PV patients is causally related to the development of erythrocytosis, with other mechanisms operating in the minority of patients with small or undetectable homozygous-mutant clones.
There are at least 2 explanations for the development of a dominant homozygous-mutant clone in PV patients. First, the dominant subclone might derive from a preexisting minor subclone after a second more centromeric mitotic recombination event, with the selective advantage reflecting extension of the region of LOH. However, there was no region of LOH that was common to the dominant subclones and absent from minor subclones (Figure 2A-C). Moreover, in some patients, the breakpoint in the dominant subclone was clearly telomeric to that of a minor subclone (eg, PV24, PV26). We therefore favor the alternative explanation that minor and dominant subclones arose independently, with the selective advantage of the latter reflecting acquisition of additional genetic or epigenetic changes.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the Translational Research Laboratory, Cambridge for sample collection.
Work in the laboratory of A.R.G. is supported by Leukemia and Lymphoma Research, the Wellcome Trust, the Medical Research Council, the Kay Kendall Leukemia Fund, the Cambridge NIHR Biomedical Research Center, the Cambridge Experimental Cancer Medicine Center, and Leukemia & Lymphoma Society of America. A.L.G. is a Kay Kendall junior clinical fellow. A.M.V. and P.G. were supported by Associazione Italiana per la Ricerca sul Cancro (Milano) “Special Program Molecular Clinical Oncology 5 × 1000” to Associazione Italiana per la Ricerca sul Cancro, Gruppo Italiano Malattie Mieloproliferative (project 1005). C.A.O. is supported by the German Research Foundation (DFG, OR255/1-1). P.J.C. is a Wellcome Trust senior clinical fellow.
National Institutes of Health
Authorship
Contribution: A.L.G. performed the research and wrote the paper; E.C., F.P., and Y.S. performed colony assays and genotyping; C.A.O. performed TET2 sequencing; B.B., P.G., C.N.H., J.T.R., F.S., F.B., E.L., M.F.M., J.-M.B., K.D., A.M.V., and C.B. contributed patient samples and clinical data; P.J.C. contributed significant intellectual input to the project; A.R.G. directed the research and wrote the paper; and all authors reviewed the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Anthony R. Green, Cambridge Institute for Medical Research, Hills Road, Cambridge, CB2 0XY, United Kingdom; e-mail: arg1000@cam.ac.uk.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal