Abstract
APCs are essential for innate and adaptive immunity as well as self-immune tolerance. Here, we show that the Cap'n'collar member Bach1 regulates the generation of APCs, specifically macrophages and dendritic cells, in mice. The impaired APC development in Bach1−/− mice was accompanied by defects in downstream T-cell responses and partial protection from experimental autoimmune encephalomyelitis. Genomewide analyses identified a panel of Bach1 target genes and ablation of the direct Bach1 target gene HO-1 exacerbated the impaired APC development observed in Bach1−/− mice. This was attributed to the impaired ability of HO-1−/−Bach1−/− double mutants to produce upstream APC progenitor cells, including common myeloid progenitor (CMP)–Flk2+. By contrast, we observed an increase in hematopoietic stem-progenitor cells (HSPCs) in these mice, suggesting a developmental block in the progression of HSPCs to CMP-Flk2+ and subsequently APCs.
Introduction
Abnormal development of APCs can result in immunodeficiency and autoimmune disease. APCs, which include macrophages, B cells, and dendritic cells (DCs), and are critical for mediating adaptive immunity to foreign antigens as well as inducing tolerance to self-antigens. At steady state, macrophages are generated from monocytes, and classic DCs are derived from their precursor (pre-cDCs) and common DC progenitors (CDPs).1-6 These cells are all generated from upstream macrophage-DC precursor (MDP) cells,7-9 which are themselves derived from less lineage-restricted common myeloid progenitors (CMPs).1 The entire populations of hematopoietic cells originate from pluripotent HSCs in the BM (see Figure 7A). Despite recent advances, the cellular factors that regulate the progression of HSCs to the development of APCs remain largely uncharacterized.
Cap ‘n’ collar (CNC) proteins are evolutionarily conserved factors that are known to be essential for the resolution of oxidative stress.10 In Caenorhabditis elegans, the CNC protein SKN-1 regulates longevity.11,12 In mammals, there are 6 members within the CNC family (p45, Nrf1, Nrf2, Nrf3, Bach1, and Bach2) that heterodimerize with small Maf proteins (MafK, MafG, and MafF) to direct either gene induction or repression.13 For instance, although the co-occupancy of p45 and MafK at genome elements activates transcription, binding of a Bach1 and MafK heterodimer to these elements results in gene repression.14 Many different CNC and Maf heterodimers are formed,15 causing the biology of CNC and Maf members to be complex. Although Nrf1−/− mice are anemic and have embryonic or postnatal lethality,16 ablation of p45 in mice leads to defective platelet production.17 Although deletion of Bach2 in mice caused impaired antibody class switching,18 disruption of Nrf2 resulted in a neurodegenerative disorder and autoimmune disease.19-21 Deciphering the physiologic role of some of the CNC/Maf proteins has also proven to be challenging because of the potential functional redundancy among them.22,23
The CNC/Maf network has been associated with a myriad of human disorders, including those of the skin, respiratory system, and hematopoietic system.10,15,24 Thus, drug therapy involving the CNC pathway is actively being studied in humans for cancer chemoprevention, inflammatory diseases, and autoimmune diseases (for review, see Sykiotis and Bohmann10 ). For example, the fumaric acid ester dimethylfumarate, which exerts neuroprotection by inducing Nrf2 expression, is currently in a phase 3 clinical trial as an orally active effective treatment of human multiple sclerosis with limited side effects.25,26 Thus, deciphering the functions of the individual CNC subunits will not only be crucial to unraveling the overlapping and unique functions of these proteins but also provide insights to the potential of targeting CNC proteins for therapy against relevant diseases. In particular, the CNC member Bach1 is up-regulated in fetal Down syndrome (DS) brain.27 Bach1 expression is also significantly higher in patients with DS–acute megakaryoblastic leukemia than patients without DS– acute megakaryoblastic leukemia.24 The best-known effect of Bach1 on transcriptional regulation is its repression of heme oxygenase-1 (HO-1),14,28,29 which is a pivotal enzyme for metabolism of heme for iron reutilization and resolving oxidative stress.30,31 HO-1 inhibits the development of autoimmune neuroinflammation probably by suppressing APC function and reducing the accumulation of self-reactive T cells in the CNS.32 At steady state, Bach1 directly binds to the HO-1 promoter to repress expression. During conditions of high heme concentration, heme associates with Bach1 and relieves this repressive effect and results in HO-1 up-regulation. In theory, the Bach1/HO-1 pathway serves as a feedback loop to maintain homeostasis of heme and to prevent oxidative stress.33 However, little is known about the physiologic function of the Bach1/HO-1 pathway and its potential role in disease pathology.
In this study, we examine whether Bach1 plays a role in autoimmune disease development and normal immune function. We report here that disruption of Bach1 alone results in partial protection from the development of autoimmune diseases with the use of experimental autoimmune encephalomyelitis (EAE), which is the murine model of human multiple sclerosis (MS).14 This phenotype is accompanied by defective peripheral T-cell responses in mice. However, deleting Bach1 does not appear to affect T-cell function intrinsically but rather impaired the development of certain APC subsets, specifically macrophages and DCs. We performed genomewide analyses and identified a panel of Bach1 target genes that might be important for APC development. Of these genes, HO-1 was highly up-regulated on deleting Bach1 in APCs. To determine the significance of HO-1 up-regulation, we generated Bach1−/−HO-1−/− mice. Unexpectedly, these animals were nearly depleted of macrophages and had lower numbers of DCs compared with Bach1−/− mice, indicating that the Bach1/HO-1 pathway is a key event for APC development. This phenotype of the HO-1−/−Bach1−/− mice was accompanied by a significant decrease in the numbers of upstream APC progenitor cells, including CMP-Flk2+, but an increase in hematopoietic stem-progenitor cell (HSPC) numbers, suggesting a developmental block in the progression of HSPCs to CMP-Flk2+ and subsequently APCs. Collectively, we identified the CNC member Bach1 as a new player in regulating steady state myelopoiesis, normal immune function, and autoimmune disease development, expanding the understanding of CNC-Maf biology. Our data indicate that the Bach1/HO-1 pathway provides a key signaling event for the development of stem cells into APCs.
Methods
Materials and flow cytometry
Antibodies for flow cytometry were purchased from eBioscience, BioLegend, or Santa Cruz Biotechnology. Flow cytometric analysis was performed with FACSCalibur (BD Bioscience) or MACSQuant (Miltenyi Biotec). The FloJo Version 9.3.2 software (TreeStar) was used for data analyses. GM-CSF and M-CSF were purchased from R&D Systems. PolyI:C was obtained from InvivoGene. Ovalbumin/complete Freund adjuvant (OVA/CFA) emulsions, MOG35-55/CFA emulsions, and pertussis toxin (PT) were purchased from Hooke Laboratories. Cytokine ELISA kits were purchased from eBioscience or BioLegend. OVA protein was purchased from Sigma-Aldrich.
Generation of Bach1−/− and HO-1−/−Bach1−/− mice
C57bl/6 Bach1−/− mice were rederived at the animal facility at California Institute of Technology from embryos obtained from the Igarashi laboratory.14 The progeny were further mated to in-house C57bl/6 animals. The Bach1+/− pups were bred to obtain wild-type (WT) and C57bl/6 Bach1−/− animals, and the respective genotypes were mated to generate experimental WT and Bach1−/− mice. FVB HO-1+/− mice31 were purchased from The Jackson Laboratory and crossed with C57bl/6 Bach1−/− mice. HO-1+/−Bach1+/− mice were bred, and HO-1+/−Bach1−/− animals were subsequently mated to generate HO-1−/−Bach1−/− animals.
BM reconstitution
C57bl/6 recipient animals were irradiated with 12 gray (1200 rad) of cesium. Ten million WT or Bach1−/− BM cells (BMCs) were injected into the lethally irradiated animals. Six to 8 weeks after reconstitution, the recipient mice were killed or immunized.
ELISAs
ELISAs for T-cell cytokines were performed as described by the manufacturer. For the MOG35-55–specific IgG ELISA (Bethyl Laboratories), MOG35-55 (2 μg/mL) peptide was coated onto a 96-well plate overnight. Serially diluted sera were loaded on the plate and quantified according to the protocol described by the manufacturer (Bethyl Laboratories; catalog no. E90-131).
OVA immunization and T-cell recall experiments
Mice were immunized with 200 μg of OVA protein emulsified in 200 μg of CFA subcutaneously. One month after immunization, the animals were boosted with 100 μg of OVA emulsified in incomplete Freund adjuvant (IFA) subcutaneously. Two weeks after boosting, the spleen was harvested, and red blood cells were lysed. Splenocytes were then cultured in cell media (Hyclone RPMI containing l-glutamine, 10% FBS, penicillin and streptomycin, 50μM β-mercaptoethanol) at a density of 1 million cells per milliliter in 12- or 24-well plates. Cells were treated with 10 μg/mL OVA.
EAE induction and immune cell infiltration analysis
EAE was induced by subcutaneous injection with emulsions that contained 200 μg of MOG35-55 and 400 μg of CFA and 600 ng of PT. The same amount of PT was injected 22-26 hours later. The following disease scoring system was used: 0.5 = partial limp tail, 1 = complete limp tail, 2 = wobbly walk, 2.5 = 1-leg paralysis, 3 = 2-leg paralysis, 3.5 = 3-leg paralysis, 4 = moribund, and 5 = death. One month after EAE induction, total spinal cord was harvested, stained with fluorochrome-conjugated antibodies, and subjected to flow cytometric analyses to quantify the ratio of immune cellular infiltrates. FACS plots were forward and side scatter gated for hematopoietic cells.
Generation of BM-DCs and BMMs
Cells harvested from the BM were plated in medium that contained 20 ng/mL GM-CSF. On day 4, GM-CSF–containing medium was replenished. Three days later, all the surviving cells were harvested and subjected to flow cytometric analyses. To generate BM-derived macrophages (BMMs), BM cells were cultured in media (Hyclone DMEM with 10% fetal bovine serum plus penicillin/streptomycin) supplemented with 10 ng/mL M-CSF. Six days later, the adherent macrophages were replenished with new media without M-CSF, and experiments were performed.
Microarray experiments
RNAs from WT and Bach1−/− BMMs were submitted to University of California San Francisco Microarray Core Facility for gene expression analyses. Briefly, Agilent 4 × 44K GE Mm arrays were used as platforms. Sample preparation, labeling, and array hybridizations were performed according to standard protocols from the University of California San Francisco Shared Microarray Core Facilities. Arrays were scanned with the Agilent microarray scanner, and raw signal intensities were obtained with Feature Extraction version 10.1 software (Agilent). All microarray data are available at ArrayExpress under accession no. E-MEXP-3674.
Statistical analyses
All P values were obtained with Student t tests (unpaired 2-tailed with 95% confidence interval) except for Figure 1A-B, in which 2-way ANOVA was used.
Results
Deletion of Bach1 in mice offers partial protection from development of EAE
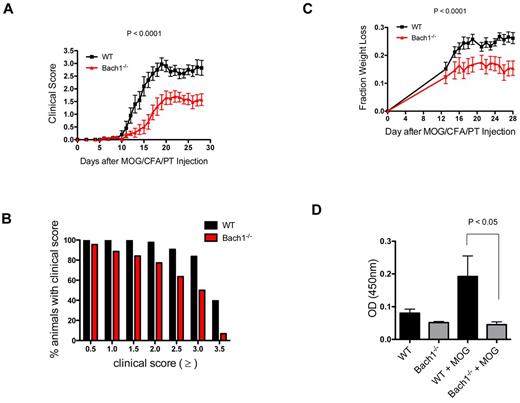
As a test of the role of Bach1 in immunity we examined its role in EAE pathogenesis. We induced EAE in WT and Bach1−/− mice14 by injecting them with the immunogenic myelin peptide MOG35-55 in CFA and PT. Standardized clinical disease scoring was used to quantify the severity of paralysis, with higher numbers indicating more severe symptoms. In contrast to Nrf2−/− mice,34 which get severe autoimmune disease, Bach1−/− mice were partially protected from EAE pathogenesis (Figure 1A). Although 40% of tested WT animals developed complete hind leg paralysis and front leg paresis (clinical score, ≥ 3.5), only 7% of Bach1−/− mice developed such severe EAE symptom (Figure 1B). Of the animals tested, 91% of WT animals developed partial hind leg paralysis (clinical score, 2.5), whereas only 64% of Bach1−/− animals exhibited this symptom. Of note, similar percentages of WT and Bach1−/− animals (100% WT and 96% Bach1−/−) developed mild EAE symptoms, marked by partial limp tail (clinical score, 0.5). Collectively, these data indicate that Bach1 does not alter the incidence of EAE development but affects the severity of the disease. Consistent with this, the decrease in EAE severity corresponded to less weight loss in Bach1−/− animals (Figure 1C); we started measuring the weight of the animals after 12 days of EAE induction when signs of disease became noticeable. Moreover, Bach1−/− mice were less capable of producing antibodies against MOG35-55 after immunization (Figure 1D), indicative of a reduced immune response in these mice.
Bach1-deficient mice exhibit decreased severity in the autoimmune disease model EAE. WT-type or Bach1−/− mice were immunized subcutaneously with MOG35-55 emulsified in CFA and injected with PT. (A) Bach1−/− mice had decreased severity of paralysis in the EAE model. After induction of EAE, clinical disease scores were measured. One of 3 independent experiments is shown; each experiment contained ≥ 7 animals per genotype. Two-way ANOVA was used for P value. (B) Bach1−/− mice were protected from severe paralysis after EAE induction. The graph shows the percentage of animals that reach the corresponding clinical score. The data were generated with > 40 WT animals and Bach1−/− animals. (C) Bach1−/− mice were less sick after EAE induction. The weights of the mice were taken at the indicated times after EAE induction and expressed as fraction weight loss. Two-way ANOVA, P < .001 (WT N = 28; Bach1−/− N = 29). (D) Bach1−/− animals produced lower amount of IgG antibodies against MOG35-55. Serum from mice (N = 10) before and 2 weeks after EAE induction were subjected to ELISA analysis to quantify the relative amount of MOG35-55–specific antibody produced, expressed as OD450 nm in the plot. All graphs are represented with SEM.
Bach1-deficient mice exhibit decreased severity in the autoimmune disease model EAE. WT-type or Bach1−/− mice were immunized subcutaneously with MOG35-55 emulsified in CFA and injected with PT. (A) Bach1−/− mice had decreased severity of paralysis in the EAE model. After induction of EAE, clinical disease scores were measured. One of 3 independent experiments is shown; each experiment contained ≥ 7 animals per genotype. Two-way ANOVA was used for P value. (B) Bach1−/− mice were protected from severe paralysis after EAE induction. The graph shows the percentage of animals that reach the corresponding clinical score. The data were generated with > 40 WT animals and Bach1−/− animals. (C) Bach1−/− mice were less sick after EAE induction. The weights of the mice were taken at the indicated times after EAE induction and expressed as fraction weight loss. Two-way ANOVA, P < .001 (WT N = 28; Bach1−/− N = 29). (D) Bach1−/− animals produced lower amount of IgG antibodies against MOG35-55. Serum from mice (N = 10) before and 2 weeks after EAE induction were subjected to ELISA analysis to quantify the relative amount of MOG35-55–specific antibody produced, expressed as OD450 nm in the plot. All graphs are represented with SEM.
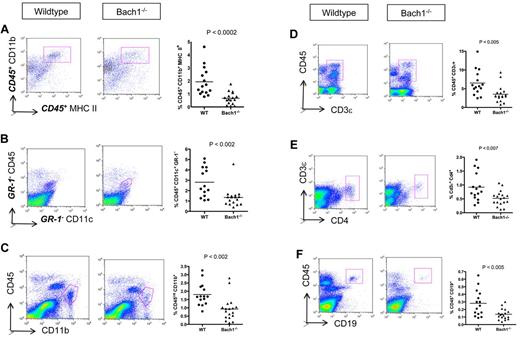
The pathogenesis of EAE initiates with immune cell infiltration into the CNS. The lower inflammatory condition (Figure 1D) in Bach1−/− mice suggested that these animals might be protected from EAE as a result of reduced penetration of immune cells into the CNS. To assess this, we harvested spinal cord from diseased animals and used flow cytometry to quantify the percentage of infiltrating hematopoietic cells. Consistent with the lower disease severity, fewer APCs (CD45+ CD11b+ MHC II+) were found in the CNS of Bach1−/− EAE animals (Figure 2A). This was a consequence of both less infiltration of peripheral DCs (Figure 2B) and fewer resident CNS microglia (Figure 2C), which proliferate on EAE development.35 In parallel to lower APCs, fewer infiltrating T and B cells were observed in the CNS of these animals (Figure 2D-F). Thus, during pathogenesis of EAE, ablation of Bach1 partially protects the CNS from immune cell infiltration and disease development.
Bach1−/− EAE mice display less inflammation in the CNS than WT animals. Spinal cords of mice were harvested 1 month after EAE induction, and immune cell infiltrates were quantified by FACS. The left graph represents the FACS plots after forward scatter and side scatter gating, and the right plot shows the compiled data of the relative percentage of corresponding immune cells infiltrated into the spinal cord of the EAE animals. (A) Lower APCs (CD45+ CD11b+ MHC II+) in Bach1−/− mice induced with EAE. Displayed FACS plots are CD45+ gated. (B) DCs were distinguished as CD45+ CD11c+ GR-1− cells. Displayed FACS plots are GR-1− gated. (C) Microglia population was marked as CD45int CD11b+ population. (D) Infiltration of pan T cells in the spinal cord were identified as CD45+ CD3ϵ+ cells. (E) Th infiltrates were distinguished as CD3e+CD4+ cells (cell population in pink box). (F) B cells in the spinal cord were identified as CD45+ CD19+ cells (cell population in pink box). Bolded letters of the surface markers denote that the plot shown are after gating to that particular marker. Each group contains ≥ 15 animals. Unpaired 2-tail Student t test performed for all the panels to obtain P value. The horizontal bars on each graph represents the average percentage.
Bach1−/− EAE mice display less inflammation in the CNS than WT animals. Spinal cords of mice were harvested 1 month after EAE induction, and immune cell infiltrates were quantified by FACS. The left graph represents the FACS plots after forward scatter and side scatter gating, and the right plot shows the compiled data of the relative percentage of corresponding immune cells infiltrated into the spinal cord of the EAE animals. (A) Lower APCs (CD45+ CD11b+ MHC II+) in Bach1−/− mice induced with EAE. Displayed FACS plots are CD45+ gated. (B) DCs were distinguished as CD45+ CD11c+ GR-1− cells. Displayed FACS plots are GR-1− gated. (C) Microglia population was marked as CD45int CD11b+ population. (D) Infiltration of pan T cells in the spinal cord were identified as CD45+ CD3ϵ+ cells. (E) Th infiltrates were distinguished as CD3e+CD4+ cells (cell population in pink box). (F) B cells in the spinal cord were identified as CD45+ CD19+ cells (cell population in pink box). Bolded letters of the surface markers denote that the plot shown are after gating to that particular marker. Each group contains ≥ 15 animals. Unpaired 2-tail Student t test performed for all the panels to obtain P value. The horizontal bars on each graph represents the average percentage.
Bach1 indirectly regulates T cell–mediated immune responses through controlling the steady state development of APCs
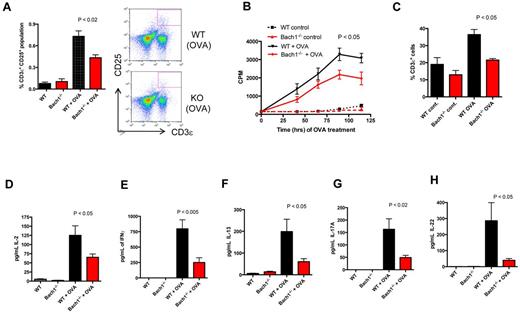
Our finding that deletion of Bach1 lowered infiltration of immune cell into the CNS during EAE pathogenesis, which is a T cell–mediated disease, provided the impetus for us to examine whether Bach1 regulates T-cell effector responses in the periphery. To test this, we immunized WT and Bach1−/− mice with OVA, harvested the splenocytes, and restimulated the cultured splenocytes with vehicle or OVA. Indeed, we observed that OVA stimulation of the Bach1−/− splenocytes induced activation of fewer T cells (Figure 3A) accompanied by impaired T-cell proliferation (Figure 3B) and expansion (Figure 3C). Consistently, we detected in activated splenocytes lower amounts of IL-2, IFNγ, IL-13, and IL-17A cytokines (Figure 3D-H), which are produced by Th1, Th2, and Th17. These data indicate that deletion of Bach1 lowers general CD4+ T-cell responses, including those of Th1, Th2, and Th17.
Defective Th response on ablation of Bach1. WT or Bach1−/− mice were immunized subcutaneously with 100 μg of OVA/CFA. One month after immunization, the animals were boosted with 100 μg in IFA. Splenocytes were isolated, equal numbers were plated and cultured with OVA (10 μg/mL). (A) Bach1−/− splenocytes are defective in T-cell activation. After 2 days of OVA treatment, splenocytes were subjected to flow cytometric analysis to determine the percentage of T cells that expressed the activation marker (CD25) after OVA induction. The graph shows the average percentage of CD3ϵ+ CD25+ population in the splenocytes. P < .05. KO indicates knockout. (B) Bach1−/− splenocytes are defective in antigen-specific–stimulated proliferation. Splenocytes were pulsed with tritiated (3H) thymidine for 16-24 hours. The incorporated radiolabeled thymidine in the cells was quantified in scintillation fluid and measured as counts per minute (CPM). The time on the x-axis indicates the hours of OVA stimulation, which also includes the 3H thymidine pulse time. Two-way ANOVA of WT OVA versus Bach1−/− OVA, P < .001. (C) Impaired OVA-induced T-cell expansion on deletion of Bach1. Splenocytes were cultured for 60 hours, and flow cytometry was used to quantify the percentage of CD3ϵ T cells. (D-H) Defective production of T-cell cytokines on OVA restimulation in Bach1−/− splenocytes. After OVA restimulation of culture splenocytes for 3 days, ELISAs were performed with the supernatant fluid to measure cytokine production. Three independent experiments were performed, and each experiment contained 4-12 mice per genotype. WT OVA versus Bach1−/− OVA, P < .05. All plots are expressed with SEM.
Defective Th response on ablation of Bach1. WT or Bach1−/− mice were immunized subcutaneously with 100 μg of OVA/CFA. One month after immunization, the animals were boosted with 100 μg in IFA. Splenocytes were isolated, equal numbers were plated and cultured with OVA (10 μg/mL). (A) Bach1−/− splenocytes are defective in T-cell activation. After 2 days of OVA treatment, splenocytes were subjected to flow cytometric analysis to determine the percentage of T cells that expressed the activation marker (CD25) after OVA induction. The graph shows the average percentage of CD3ϵ+ CD25+ population in the splenocytes. P < .05. KO indicates knockout. (B) Bach1−/− splenocytes are defective in antigen-specific–stimulated proliferation. Splenocytes were pulsed with tritiated (3H) thymidine for 16-24 hours. The incorporated radiolabeled thymidine in the cells was quantified in scintillation fluid and measured as counts per minute (CPM). The time on the x-axis indicates the hours of OVA stimulation, which also includes the 3H thymidine pulse time. Two-way ANOVA of WT OVA versus Bach1−/− OVA, P < .001. (C) Impaired OVA-induced T-cell expansion on deletion of Bach1. Splenocytes were cultured for 60 hours, and flow cytometry was used to quantify the percentage of CD3ϵ T cells. (D-H) Defective production of T-cell cytokines on OVA restimulation in Bach1−/− splenocytes. After OVA restimulation of culture splenocytes for 3 days, ELISAs were performed with the supernatant fluid to measure cytokine production. Three independent experiments were performed, and each experiment contained 4-12 mice per genotype. WT OVA versus Bach1−/− OVA, P < .05. All plots are expressed with SEM.
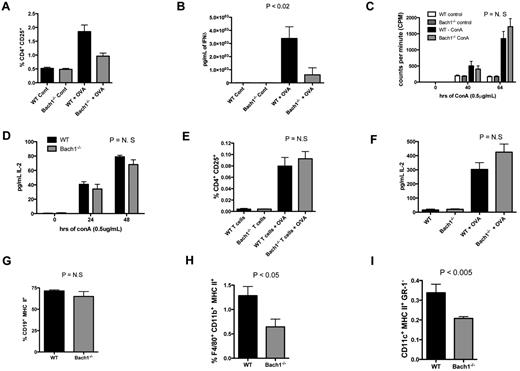
To delineate whether Bach1-mediated T-cell response is intrinsic to the hematopoietic compartment, WT and Bach1−/− BMCs were reconstituted into lethally irradiated C57bl/6 recipient mice, which were then immunized with OVA. Indeed, fewer CD4+ T cells were activated (Figure 4A; supplemental Figure 1A, available on the Blood Web site; see the Supplemental Materials link at the top of the online article) and lower amounts of T-cell cytokines (Figure 4B) were produced from the splenocytes harvested from animals reconstituted with Bach1−/− BMCs. Importantly, treatment of WT and Bach1−/− splenocytes with the direct T cell–specific activation ligand ConA led to similar proliferation of T cells (Figure 4C) and production of equal amounts of cytokines (Figure 4D). In addition, CD4+ T cells purified from OVA-immunized WT or Bach1−/− mice were similarly responsive to OVA activation when cocultured with naive total WT splenocytes, which served as a source of APCs. This was indicated by the percentage of CD4+CD25+-activated T cells and IL-2 secretion (Figure 4E-F). Collectively, these data indicated that Bach1 regulates T-cell responses through its function within the hematopoietic compartment but does not function intrinsically in T cells for activation. This suggested that Bach1 regulates upstream events to modulate T-cell activation and responses. To further determine the mechanism by which Bach1 regulates T-cell immunity, we determined whether disruption of Bach1 had any effect on APCs. Interestingly, although B-cell numbers were similar in Bach1−/− mice (Figure 4G; supplemental Figure 1B), fewer APCs (macrophages and DCs; Figure 4H-I; supplemental Figure 1C-D) were observed in these animals. Hence, Bach1 appears to be needed for production of normal numbers of specific types of APCs and thus regulates T-cell responses indirectly.
Impaired T-cell responses in C57bl/6 recipient animals reconstituted with BMCs from Bach1−/− donors. Lethally irradiated C57bl/6 recipients reconstituted with BMCs from WT or Bach1−/− donors were immunized with OVA/CFA, and splenocytes were cultured in the presence or absence of OVA. After 3 days in culture, splenocytes were subjected to (A) flow cytometric analyses (see also supplemental Figure 1A) to quantify the percentage of CD4+ cells that expressed the CD25 T-cell activation marker and (B) the supernatant fluids were examined for the amount of IFNδ with the use of ELISAs. Student t test P < .05 for the OVA-treated samples. (C) Total splenocytes from WT or Bach1−/− animals were stimulated with concanavalin A (ConA) and pulsed with tritiated thymidine for the last 16-22 hours. (D) The amount of IL-2 secreted by T cells in total splenocytes after stimulation with ConA was determined by ELISA. (E-F) WT and Bach1−/− mice were immunized with OVA/CFA and boosted with OVA/IFA. Splenic CD4+ T cells from these animals were purified 2 weeks later and cocultured with naive total WT splenocytes in the absence or presence of OVA for 72 hours. The percentage of activated CD4+ T cells (CD4+ CD25+) was measured by flow cytometry, (E) and the amount of secreted IL-2 was quantified by ELISA (F). Splenocytes from animals that had undergone immunization with OVA/CFA were subjected to flow cytometric analyses to examine the percentage of B cells (G), macrophages (H), and DCs (I). See also supplemental Figure 1B, C, and D for flow cytometric plots for panels G, H, and I, respectively. The data represent averages of ≥ 3 mice per group.
Impaired T-cell responses in C57bl/6 recipient animals reconstituted with BMCs from Bach1−/− donors. Lethally irradiated C57bl/6 recipients reconstituted with BMCs from WT or Bach1−/− donors were immunized with OVA/CFA, and splenocytes were cultured in the presence or absence of OVA. After 3 days in culture, splenocytes were subjected to (A) flow cytometric analyses (see also supplemental Figure 1A) to quantify the percentage of CD4+ cells that expressed the CD25 T-cell activation marker and (B) the supernatant fluids were examined for the amount of IFNδ with the use of ELISAs. Student t test P < .05 for the OVA-treated samples. (C) Total splenocytes from WT or Bach1−/− animals were stimulated with concanavalin A (ConA) and pulsed with tritiated thymidine for the last 16-22 hours. (D) The amount of IL-2 secreted by T cells in total splenocytes after stimulation with ConA was determined by ELISA. (E-F) WT and Bach1−/− mice were immunized with OVA/CFA and boosted with OVA/IFA. Splenic CD4+ T cells from these animals were purified 2 weeks later and cocultured with naive total WT splenocytes in the absence or presence of OVA for 72 hours. The percentage of activated CD4+ T cells (CD4+ CD25+) was measured by flow cytometry, (E) and the amount of secreted IL-2 was quantified by ELISA (F). Splenocytes from animals that had undergone immunization with OVA/CFA were subjected to flow cytometric analyses to examine the percentage of B cells (G), macrophages (H), and DCs (I). See also supplemental Figure 1B, C, and D for flow cytometric plots for panels G, H, and I, respectively. The data represent averages of ≥ 3 mice per group.
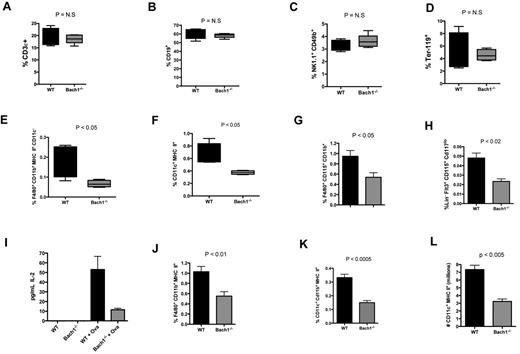
To more precisely characterize the numbers of various cell types in the knockout animals, splenocytes from naive WT and Bach1−/− mice were subjected to flow cytometric analyses. The total number of splenic leukocytes between the 2 groups of animals was similar (supplemental Figure 1E). Although the percentage of T cell (CD3ϵ+), B cells (CD19+), natural killer cells (NK1.1+ CD49b+), and erythrocytes (Ter119+; Figure 5A-D; supplemental Figure 2A-D) were similar between the 2 groups, the fractions of macrophages (MHC II+ CD11c− CD11b+ F4/80+; Figure 5E; supplemental Figure 2E) and CD11c+ DCs (Figure 5F; supplemental Figure 2F) were significantly lower in the Bach1−/− animals. Furthermore, the populations of macrophage-committed (CD11b+ F4/80+ CD115+; Figure 5G; supplemental Figure 2G) and DC-committed precursors5,6 were lower in the BM compartment of the mutant animals (Figure 5H; supplemental Figure 2H).
Bach1 regulates steady state development of macrophages and DCs through its intrinsic function in the BM compartment. White blood cells (WBCs) harvested from splenocytes of age-matched adult animals were subjected to FACS analyses to quantify the percentage population of (A) T lymphocytes (CD3ϵ+), (B) non–plasma B cells (CD19+), (C) natural killer cells (NK1.1+, CD49b+), (D) erythrocytes (Ter-119+), (E) monocyte/macrophages (F4/80+ CD11b+ CD11c− MHC II+), and (F) DCs (CD11c+ MHC II+). (G-H) Bach1 regulates the development of DC-precursors and monocytes. WT and Bach1−/− BM cells were subjected to flow cytometric analyses to quantify the percentage of DC-precursors (G) and monocytes (H). LIN contains a cocktail of Ter-119, CD11b, B220, GR-1, and CD3ϵ. (I) Equal numbers of splenocytes from naive WT or Bach1−/− mice were cocultured with purified OTII CD4+ T cells in the absence or presence of OVA for 72 hours. The amount of secreted IL-2 was quantified by ELISA. WT versus Bach1−/− OVA-treated samples: P < .05. (J) Macrophages and (K) DCs (right panel) in the BM of lethally irradiated C57bl/6 recipients reconstituted with BMCs from WT or Bach1−/− donors. (L) Bach1 influences the development of BM cells into DCs in vitro. Equal numbers of WT and Bach1−/− BMCs were cultured with 20 ng/mL GM-CSF. Total number of DCs (CD11c+ MHC II+) generated at day 7 was calculated by cell counting and flow cytometric analyses. All data represent experiments containing ≥ 3 animals per genotype. The graphs are plotted with SEM.
Bach1 regulates steady state development of macrophages and DCs through its intrinsic function in the BM compartment. White blood cells (WBCs) harvested from splenocytes of age-matched adult animals were subjected to FACS analyses to quantify the percentage population of (A) T lymphocytes (CD3ϵ+), (B) non–plasma B cells (CD19+), (C) natural killer cells (NK1.1+, CD49b+), (D) erythrocytes (Ter-119+), (E) monocyte/macrophages (F4/80+ CD11b+ CD11c− MHC II+), and (F) DCs (CD11c+ MHC II+). (G-H) Bach1 regulates the development of DC-precursors and monocytes. WT and Bach1−/− BM cells were subjected to flow cytometric analyses to quantify the percentage of DC-precursors (G) and monocytes (H). LIN contains a cocktail of Ter-119, CD11b, B220, GR-1, and CD3ϵ. (I) Equal numbers of splenocytes from naive WT or Bach1−/− mice were cocultured with purified OTII CD4+ T cells in the absence or presence of OVA for 72 hours. The amount of secreted IL-2 was quantified by ELISA. WT versus Bach1−/− OVA-treated samples: P < .05. (J) Macrophages and (K) DCs (right panel) in the BM of lethally irradiated C57bl/6 recipients reconstituted with BMCs from WT or Bach1−/− donors. (L) Bach1 influences the development of BM cells into DCs in vitro. Equal numbers of WT and Bach1−/− BMCs were cultured with 20 ng/mL GM-CSF. Total number of DCs (CD11c+ MHC II+) generated at day 7 was calculated by cell counting and flow cytometric analyses. All data represent experiments containing ≥ 3 animals per genotype. The graphs are plotted with SEM.
To determine whether the decrease in macrophage and DC populations in Bach1−/− mice was responsible for the lowered T-cell responses (Figure 4), we cocultured splenocytes OTII CD4+ T cells in the presence of OVA. A given number of Bach1−/− splenocytes were less able than WT cells to induce an OVA OTII CD4+ T-cell response, which is consistent with Bach1−/− mice having fewer APCs (Figure 5I). Thus, our data indicate that Bach1 regulates the steady state level of specific populations of APCs, limiting the magnitude of T-cell responses.
Bach1 regulates development of APCs in a cell-autonomous manner
To investigate whether Bach1 functions within the hematopoietic compartment to activate APC development, we reconstituted lethally irradiated C57bl/6 WT recipient mice with WT or Bach1−/− BMCs. The animals reconstituted with Bach1−/− BMCs exhibited fewer macrophages and DCs in their spleens than animals that received WT BMCs (Figure 5J-K). To test whether Bach1 functions in other nonhematopoietic compartments to mediate DC development, WT and Bach1−/− lethally irradiated mice were reconstituted with WT BMCs. Both recipient groups displayed a similar percentage of splenic macrophages and DCs (data not shown). Thus, Bach1 influences the development of these APCs by functioning within the hematopoietic compartment and not through other nonhematopoietic tissues. To examine whether Bach1 modulates APC development in a cell-autonomous manner, BMCs were isolated and treated with GM-CSF to generate DCs cells in vitro.36 Similar to our in vivo finding, BMCs harvested from Bach1−/− mice produced < 50% of the DCs generated by WT cells (Figure 5L). These in vitro experiments combined with our in vivo analyses imply that Bach1 is directly involved in the development of a subset of APCs through a cell-autonomous mechanism.
Identification of Bach1 targets
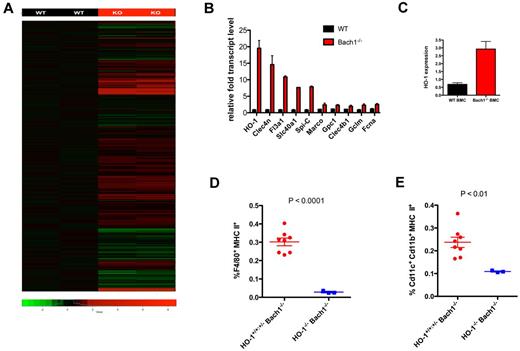
To provide insights into the mechanism and molecular pathways by which Bach1 regulates APC development, we performed whole-genome gene expression microarray to identify gene targets regulated by Bach1 in BMMs. With the use of this unbiased approach, we identified > 300 genes differentially expressed in WT versus Bach1−/− BMMs (Figure 6A; supplemental Figure 3). We validated by quantitative PCR a panel of genes up-regulated on deletion of Bach1−/− (Figure 6B). In our microarrays, we found that the known direct Bach1 target HO-1 was the most highly up-regulated in Bach1−/− BMMs.14,28-31 In addition, we also isolated previously unknown Bach1 targets, including Clec4n and Fl3a1, as well as Slc40a1/Fpn1. Slc40a1/Fpn1 was previously found to be concomitantly up-regulated with HO-1 after erythrophagocytosis.37 Because HO-1 was identified in our microarrays as the most affected by Bach1 ablation, we next examined whether HO-1 could also be regulated by Bach1 in total primary BMCs. Indeed, we found that Bach1−/− BMCs expressed significantly higher levels of HO-1 transcript (Figure 6C). Collectively, we identified a panel of Bach1 target genes that may be involved in Bach1-mediated APC development.
HO-1 ablation exacerbates impaired APC development in Bach1−/− mice. (A) RNA from WT and Bach1−/− BMMs were harvested and reverse transcribed, and cDNA was subjected for microarray analyses. Genes highlighted in red or green represent those up- or down-regulated, respectively, in Bach1−/− BMMs. See also supplemental Figure 3. (B) Quantitative PCR analyses to verify Bach1 target genes identified in the microarrays. WT and Bach1−/− BMMs were harvested for gene analyses. Relative transcript levels were normalized to the house keeping gene Rpl19. (C) Bach1 regulates HO-1 in BM cells. Transcript level of HO-1 was assessed from samples harvested from WT and Bach1−/− BMCs from 3 separate animals. (D-E) BM cells were isolated from mice of the indicated genotype and subjected to flow cytometric analyses to measure the percentage of (D) macrophages and (E) DCs. Each dot represents an individual animal. Red graph contains 3 HO-1+/+Bach1−/− and 5 HO-1+/−Bach1−/− mice. Blue represents 3 HO-1+/−Bach1−/− mice. All graphs are plotted with SEM. The horizontal bars on each graph represents the average percentage.
HO-1 ablation exacerbates impaired APC development in Bach1−/− mice. (A) RNA from WT and Bach1−/− BMMs were harvested and reverse transcribed, and cDNA was subjected for microarray analyses. Genes highlighted in red or green represent those up- or down-regulated, respectively, in Bach1−/− BMMs. See also supplemental Figure 3. (B) Quantitative PCR analyses to verify Bach1 target genes identified in the microarrays. WT and Bach1−/− BMMs were harvested for gene analyses. Relative transcript levels were normalized to the house keeping gene Rpl19. (C) Bach1 regulates HO-1 in BM cells. Transcript level of HO-1 was assessed from samples harvested from WT and Bach1−/− BMCs from 3 separate animals. (D-E) BM cells were isolated from mice of the indicated genotype and subjected to flow cytometric analyses to measure the percentage of (D) macrophages and (E) DCs. Each dot represents an individual animal. Red graph contains 3 HO-1+/+Bach1−/− and 5 HO-1+/−Bach1−/− mice. Blue represents 3 HO-1+/−Bach1−/− mice. All graphs are plotted with SEM. The horizontal bars on each graph represents the average percentage.
Deletion of HO-1 exacerbates the impaired APC development observed in Bach1−/− mice
To investigate the mechanism by which Bach1 controls APC development, we examined the function of HO-1 up-regulated expression in the context of Bach1 ablation in hematopoietic development. We chose to study HO-1 because it has been previously identified as a direct target of Bach114,28,29 and was identified as the most affected gene in our microarrays by Bach1 deletion (Figure 6A-B). To this end, we generated HO-1−/−Bach1−/− mice by breeding Bach1−/− with HO-1+/− mice31 and intercrossing their subsequent progeny. Breeding of HO-1+/−Bach1+/− parent pairs did not produce any HO-1−/−Bach1−/− animals (36 pups genotyped), and breeding of HO-1+/−Bach1−/− pairs resulted in < 2% of pups being HO-1−/−Bach1−/−, which is much lower than the expected 25% Mendelian distribution (chi square P < .001). In the BM compartment, we detected almost no macrophages in the HO-1−/−Bach1−/− mice (Figure 6D). The compound HO-1−/−Bach1−/− mice had even fewer DCs than Bach1−/− animals (Figure 6E). It was reported that the generation of HO-1−/− mice was more successful in FVB than the C57bl/6 background.38 Thus, we attempted to generate FVB HO-1−/− mice through breeding heterozygotes; however, our breeding of HO-1+/− pairs did not produce any HO-1−/− pups (> 90 pups genotyped), precluding us from being able to examine APC development in mice with only HO-1 deletion. Nevertheless, our data indicate that the Bach1/HO-1 pathway is essential for macrophage development in the BM and necessary for optimal development of DCs.
In addition, we also assessed whether the Bach1/HO-1 pathway regulates the development of other hematopoietic cells. We found that the HO-1−/−Bach1−/− mice had similar numbers of T-cells (CD4+ CD3ϵ+, CD8α+ CD3ϵ+), natural killer cells (NK1.1+ GR-1−), B cells (CD19+ GR-1−), and GR-1+ myeloid cells as the Bach1−/− mice (supplemental Figure 4), which have normal development of these cells compared with WT animals as described above (Figure 5). This indicates that the Bach1/HO-1 pathway regulates APC development without affecting these immune cells.
The Bach1/HO-1 pathway influences the development of APC precursors and multipotent myeloid progenitors
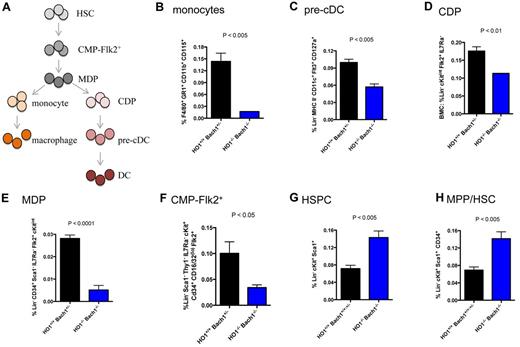
To further probe the mechanism by which Bach1/HO-1 pathway orchestrates APC development, we assessed whether Bach1/HO-1 regulates APC precursor cells (see Figure 7A). We examined and found that ablating Bach1 and HO-1 resulted in impaired development of monocytes (Figure 7B), precursors of macrophages.5,6 The HO-1−/−Bach1−/− mice were also defective in developing precursors of the DC-lineage. The pre-cDCs (Figure 7C) and the more upstream common DC progenitors (CDPs; Figure 7D) were both reduced in HO-1−/−Bach1−/− mice. Monocytes and CDPs are generated from the macrophage-DC precursor (MDPs; Figure 7A).7-9 Further upstream of MDPs in APC development are Flk2+ CMPs (CMP-Flk2+) that have multipotent myeloid-forming potential.39 Strikingly, HO-1−/−Bach1−/− mice had fewer numbers of both MDPs and CMP-Flk2+ (Figure 7E-F), indicating that the Bach1/HO-1 pathway controls the production of APC by controlling the development of multipotent myeloid progenitors. In contrast, the HO-1−/−Bach1−/− mice have an increase in their fraction of Lin− cKit+ Sca1+ pluripotent HSPCs (Figure 7G). Further analyses indicated that these mice had an elevated percentage of MPP and ST-HSCs (Figure 7H). Collectively, our data suggest that ablation of the Bach1/HO-1 signaling pathway results in a developmental block from the progression of MPP/ST-HSCs to CMP-Flk2+ and subsequently APCs. Thus, we conclude that the Bach1/HO-1 pathway is an important event for committing pluripotent stem-progenitor cells to develop into APCs.
BM cells were harvested from adult mice and subjected to flow cytometric analyses. (A) Development of macrophages and DCs from HSCs. HSCs give rise to CMP-Flk2+. Macrophage-DC-restricted progenitors (MDPs) can generate both macrophages and DCs, whereas common DC progenitors (CDPs) are restricted to formation of DCs. The panels correspond to the following cells: (B) monocytes, (C) pre-cDCs, (D) CDPs, (E) MDPs, (F) CMP-Flk2+ cells, (G) HSPCs, and (H) multipotent progenitor (MPP) or short-term hematopoietic stem cells (ST-HSCs). Each plot represents ≥ 3 mice. The graphs are plotted with SEM.
BM cells were harvested from adult mice and subjected to flow cytometric analyses. (A) Development of macrophages and DCs from HSCs. HSCs give rise to CMP-Flk2+. Macrophage-DC-restricted progenitors (MDPs) can generate both macrophages and DCs, whereas common DC progenitors (CDPs) are restricted to formation of DCs. The panels correspond to the following cells: (B) monocytes, (C) pre-cDCs, (D) CDPs, (E) MDPs, (F) CMP-Flk2+ cells, (G) HSPCs, and (H) multipotent progenitor (MPP) or short-term hematopoietic stem cells (ST-HSCs). Each plot represents ≥ 3 mice. The graphs are plotted with SEM.
Discussion
Here, we found that optimal developmental progression from pluripotent stem cells to macrophages and DCs requires Bach1/HO-1 signaling. First, we found that Bach1, a member of the CNC protein family, contributes to the development of APCs, specifically macrophages and DCs. The impaired APC development in Bach1-deficient mice was accompanied by defects in downstream Th responses, indicating the functional significance of Bach1 in immune responses. Importantly, and consistent with a dampened T-cell response, ablation of Bach1 in mice resulted in partial protection of these animals from development of the T cell–mediated autoimmune disorder EAE. Thus, we have defined a new player in immune function and shown that Bach1 influences hematopoiesis and immunity in a manner that affects the development of autoimmune disease. We next identified a panel of Bach1 target genes and examined whether the most highly regulated gene, HO-1, serves as an effector in APC development. Through these analyses, we found that Bach1/HO-1 signaling is critical for DC and macrophage development, indicating that this pathway is important for maintaining steady state hematopoiesis in addition to the previously predicted heme homeostasis.33 Mechanistically, because HO-1−/−Bach1−/− double mutants had a decreased population of early APC progenitor cells (CMP-Flk2+) accompanied by an increased fraction of the further upstream HSPCs, it suggests that these mice exhibit a developmental block in the progression from HSPCs to CMP-Flk2+ and subsequently APCs. Thus, we infer that the Bach1/HO-1 pathway regulates a key signaling event for committing stem cells to develop into APCs.
Advances have been made toward the identification of precursor cells that are capable of developing into APCs. It has been shown that DCs are generated from Lin−Flt3+CD115+CD117 (cKit)lo CDP cells.6 Macrophages and DCs are derived from common precursors coined MDP.7-9 MDPs are probably derived from upstream CMP-Flk2+ cells, which can be differentiated into myeloid cells but are restricted from forming erythroid cells.39 CMP-Flk2+ cells themselves are produced from pluripotent HSCs. The biologic program that directs CDPs, MDPs, and CMP-Flk2+ development and the signaling pathways that commit HSCs to differentiate into CMP-Flk2+ have not been well defined. We report here that Bach1/HO-1 signaling contributes importantly to the generation of CDPs, MDPs, and CMP-Flk2+. Further, a decrease in the number of these cells in HO-1−/−Bach1−/− mice was accompanied by an increase in HSC population, suggesting that Bach1/HO-1 probably provides a positive signal for either committing HSCs toward CMP-Flk2+ lineage or negative signal to inhibit HSCs from differentiation.
CNC proteins and their interaction partners small Mafs are widely studied in part because of their potentially extensive therapeutic interest, including in MS, aging, respiratory diseases, and cancer. Although advances toward understanding the involvement of Bach1 in cytoprotection during liver and myocardial ischemia have been made,40,41 the biologic role of the CNC member Bach1 is still largely unknown. Here, we show that, in addition to Bach1-mediated normal programming of APC development, it plays a role in generating optimal immunity and autoimmune-related pathology. Indeed, Bach1−/− mice are impaired in launching a T cell–mediated response, which is probably attributed to their abnormal APC development because Bach1 does not appear to function intrinsically in T cells. Of importance, the deletion of Bach1−/− partially protects animals from developing EAE, connecting Bach1 function to the severity of autoimmune diseases. It has been shown that Nrf2, a relative of Bach 1, inhibits the maturation of DCs in vitro42 and protects humans from MS development.25,26 Potentially, Nrf2 and Bach1 might serve as counterparts in the CNC/Maf regulatory network, regulating the homeostatic balance of DC development and MS pathogenesis. Moreover, it is important to highlight that deletion of Bach1 did not completely ablate APC development, suggesting that other CNC and/or small Maf subunits might have compensatory or redundant functions with Bach1. Potentially, up-regulation of HO-1 might protect APC development in Bach1−/− mice because deleting HO-1 in these animals exacerbated the decrease in DC and macrophage numbers. Alternatively, up-regulation of other target genes, such as Spi-C, which has been found to regulate the genesis of red pulp macrophages,43 might protect APC development in Bach1−/− mice. During the preparation of this manuscript, it was shown that ablating HO-1 resulted in diminished number of macrophages in the spleen.44 In that study, it was found that deleting HO-1 in BM-derived macrophages resulted in toxicity of these cells on red blood cell phagocytosis, probably as a result of heme-overloaded toxicity. In our study, we found that the impaired APC development is also associated with the generation of their progenitors. Thus, it appears that HO-1 controls the number of APCs at a stage before as well as after development into mature APCs. Collectively, we also identified a panel of previously unknown Bach1 targets. It will be interesting in the future to examine whether those genes also play a role in APC development. Potentially, Bach1 may regulate distinct or overlapping subsets of those genes for DC versus macrophage development.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Kazuhiko Igarashi for sharing the Bach1−/− mice and are grateful to Alex Balazs and Michael Bethune for helpful critiques of the manuscript.
This work was supported by the National Institutes of Health (grants 5P01CA132681-02 and 5R01AI093531-02 to D.B. and grant F32 CA139883 to A.Y.-L.S.).
National Institutes of Health
Authorship
Contribution: A.Y.-L.S., Y.G.-F., A. Minisandram, A. Martin, and K.T. performed the experiments; A.Y.-L.S., M.B., and D.B. conceived the study; A.Y.-L.S., Y.G.-F., A. Mehta, A.Y.-L.S., K.T., M.B., and D.B. provided crucial reagents; A.Y.-L.S. and D.B. wrote the manuscript; and all authors provided input on the manuscript.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: David Baltimore, California Institute of Technology, 1200 East California Boulevard MC 147-75, Pasadena, CA 90227; e-mail: baltimo@caltech.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal