Abstract
Cytotoxic lymphodepletion therapies augment antitumor immune responses. The generation and therapeutic efficacy of antitumor effector T cells (TEs) are enhanced during recovery from lymphopenia. Although the effects of lymphodepletion on naive T cells (TNs) and TEs have been studied extensively, the influence of lymphodepletion on suppressor cells remains poorly understood. In this study, we demonstrate a significant increase of CD4+CD25+Foxp3+ regulatory T cells (Tregs) in sublethally irradiated lymphopenic mice. These radio-resistant Tregs inhibited the induction of TEs in tumor-draining lymph-nodes (TDLNs) during recovery from lymphopenia. The transfer of TNs into lymphopenic tumor-bearing mice resulted in some antitumor effects; however, Treg depletion after whole-body irradiation and reconstitution strongly inhibited tumor progression. Further analyses revealed that tumor-specific T cells were primed from the transferred TNs, whereas the Tregs originated from irradiated recipient cells. As in irradiated lymphopenic mice, a high percentage of Tregs was observed in cyclophosphamide-treated lymphopenic mice. The inhibition of Tregs in cyclophosphamide-treated mice significantly reduced tumor growth. These results indicate that the Tregs that survive cytotoxic therapies suppress antitumor immunity during recovery from lymphopenia and suggest that approaches to deplete radio and chemo-resistant Tregs can enhance cancer immunotherapies.
Introduction
It is well known that CD25+ T cells include naturally occurring regulatory T cells (Tregs). Takahashi and coworkers reported that CD25+ T cells constitute 5%-10% of peripheral CD4+ T cells in normal hosts; further, these authors demonstrated that the elimination of these cells resulted in the development of systemic autoimmune disease.1 They also found that forkhead box p3 (Foxp3) plays an essential role in the maintenance of the suppressive functions of Tregs.2 Foxp3 was expressed in CD4+CD25+ T cells, and the forced expression of Foxp3 converts CD4+ TNs into Tregs. Mutation of Foxp3 was first described as the cause of the X-linked recessive autoimmune and inflammatory diseases present in scurfy mice.3 Scurfy mice are deficient in the Foxp3 gene and lack Tregs.4 Knockout of Foxp3 causes a systemic autoimmune disease that is similar to the disease observed in scurfy mice.4 Thus, it is now evident that Foxp3 is necessary and sufficient for the generation and function of Tregs; in addition, Foxp3 appears to be the most specific marker for the identification of Tregs in mice.
A number of studies have demonstrated an increase of Tregs in peripheral blood, tumor sites, and tumor-draining lymph-nodes (TDLNs) of cancer patients.5 Tregs infiltration into tumor tissues has been observed in cancer patients, and increased percentages of Tregs were observed in patients with poor prognoses.6 Furthermore, the balance between TEs and Tregs in tumor tissues and among peripheral blood mononuclear cells was correlated with the prognosis of cancer patients.7,8 These findings suggested that Tregs suppress antitumor immunity and contribute to tumor progression. Indeed, recent studies demonstrated that the inhibition of Tregs enhances antitumor immunity and slows tumor progression.9
The ability of lymphodepleting cytotoxic regimens to augment antitumor immunity has been well established.10,11 Host lymphodepletion results in the extensive proliferation of TEs. In addition, TEs show 3- to 12-fold higher antitumor efficacies when adoptively transferred into lymphopenic tumor-bearing hosts.12 In clinical studies, the adoptive transfer of ex vivo–activated antitumor T cells after chemotherapy results in robust antitumor responses in melanoma patients.13 Recently, Dummer et al reported that the transfer of syngeneic TNs into irradiated or genetically lymphopenic mice inhibits tumor progression.14 Tumor-specific TEs were strongly induced by lymphodepletion and the transfer of TNs. Other investigators also showed that lymphodepletion followed by tumor-antigen vaccination increased the generation of TEs.15 Collectively, lymphodepletion augments not only the therapeutic efficacy of TEs but also the generation of TEs from TNs. Although the precise mechanisms underlying the stimulation of antitumor immunity by lymphodepletion remain unclear, many possible explanations have been proposed, including the depletion of suppressor cells, the improvement of tumor-antigen presentation, and the elimination of lymphocytes competing for activation-inducing cytokines.16-18
In this article, we report that lymphodepletion increased the percentage of CD4+CD25+Foxp3+ Tregs in whole-body irradiated mice. Although adoptive transfer of TNs after lymphodepletion showed some antitumor effects, the depletion of proliferating recipient Tregs in irradiated reconstituted mice significantly augmented the induction of TEs and inhibited tumor progression. These findings suggested that radio-resistant Tregs suppressed the development of antitumor immunity during recovery from lymphopenia.
Methods
Animals
Female C57BL/6J (B6) mice were purchased from the CLEA Laboratory, and Ly5.1 congenic B6 mice were purchased from Sankyo Labo Service. Transgenic mice expressing the green fluorescent protein (GFP) gene from Aequorea victoria were from Japan SLC. All mice were housed in a specific pathogen-free environment and used between 8 and 12 weeks of age. The experimental protocols were approved by the Niigata University Institutional Animal Care and Use Committee.
Tumors
Methylcholanthrene (MCA)205 and MCA207 are fibrosarcomas of B6 origin that were initially induced through the intramuscular injection of 3-methylcholanthrene.19 These tumor cells have been routinely passaged in vivo and were used between the fifth and eighth passages. Single-cell suspensions were prepared from solid tumors by digestion with a mixture of 0.1% collagenase, 0.01% DNase, and 2.5 U/mL hyaluronidase (Sigma-Aldrich) for 3 hours at room temperature. The cells were filtered through a 100-μm nylon mesh, washed, and suspended in Hanks balanced salt solution (HBSS) for intravenous and subdermal (SD) inoculations.
Adoptive transfer
B6 mice were lymphodepleted by sublethal irradiation with 500 cGy. On the same day, mice were reconstituted intravenously with 40 × 106 spleen cells from normal mice. Irradiated reconstituted mice were then inoculated SD with 1 × 105 MCA 205 tumor cells along the midline of the abdomen. The tumor sizes were measured in 2 perpendicular dimensions 2 to 3 times per week with digital calipers, and the tumor areas (mm2) were recorded.
Cell separation
To deplete donor cells of Thy1.2+, CD4+, CD8+, or CD25+ cells, naive spleen cells were suspended at a density of 3 × 108/mL in magnetic-activated cell sorting (MACS) buffer (0.5% bovine serum albumin in phosphate-buffered saline with 2mM ethylenediaminetetraacetic acid) and 1.0 mL of cell suspension was incubated with 100 μL of phycoerythrin (PE) anti-Thy1.2 monoclonal antibody (mAb), anti–CD4 mAb anti–CD8 mAb, or anti–CD25 mAb (BD Bioscience) for 10 minutes on ice. The cells were washed with cold MACS buffer, suspended at 3 × 108/mL in 2.4 mL of MACS buffer and then incubated at 4°C with anti-PE microbeads (Miltenyi Biotec) for 15 minutes. Thy1.2−, CD4−, CD8−, or CD25− cell populations were collected as flow through cells from MACS columns.
CD4+CD25+ Tregs cells were purified using anti–CD4 mAb-coated Dynabeads and Detachabeads (Invitrogen) followed by positive selection with PE-CD25 mAb and PE-microbeads as previously described.20
Activation of TDLNs
B6 mice were inoculated SD with 3 × 106 MCA205 cells on both flanks to stimulate TDLNs. Twelve days later, TDLNs were harvested, and single-cell suspensions were prepared mechanically. To generate TEs, TDLN cells were activated for 2 days by being cultured in 24-well plates coated with anti-CD3 mAb; the cells were subsequently expanded in complete medium containing 16 U/mL of human recombinant IL-2 (kindly supplied by Shionogi) for 3 days as previously described.21 Complete medium consisted of RPMI 1640 supplemented with 10% heat-inactivated fetal bovine serum and antibiotics. Activated TDLN cells were harvested and resuspended in HBSS for evaluation of therapeutic activity in adoptive immunotherapy.
FACS analysis and in vivo proliferation
Fluorescein isothiocyanate-conjugated mAb against CD25 (PC61), BrdU (3D4), PE-conjugated mAb against CD4 (RM4-5), CD8 (53-6.7), CD25 (PC61), IFN-γ (XMG1.2), Cy-Chrome–conjugated mAb against CD4 (RM4-5), CD8 (53-6.7), Ki-67 (B56), isotype-matched mAb, fluorescein isothiocyanate–annexin V, and 7-AAD were purchased from BD Bioscience. PE–anti-Foxp3 (FJK-16s) staining kit was purchased from eBioscience. Single-cell suspensions were labeled according to manufacturer's protocol, and analyzed using a FACSCalibur (BD Bioscience) flow cytometer. For a BrdU incorporation assay, irradiated mice were injected intraperitoneally with 1 mg of BrdU (Sigma-Aldrich) for 3 consecutive days before harvest. For analysis of in vivo proliferation, donor spleen T cells were labeled with CFSE (carboxyfluorescein diacetate succinimidyl ester; Molecular Probes). Briefly, splenic TNs were suspended at 1 × 107 /mL and incubated with CFSE in HBSS for 10 minutes at 37°C. Labeling was halted by adding ice-cold HBSS, and the cells were washed twice with HBSS before being transferred into irradiated mice.
Intracellular IFN-γ staining
Intracellular IFN-γ staining was performed as previously described.22 Briefly, T cells were stimulated with either MCA205 or MCA207 tumor cells prepared from solid tumor tissues at a 1:1 ratio. Positive control cells were stimulated with immobilized anti-CD3 mAb (145-2C11). Brefeldin A at a concentration of 10 μg/mL (Sigma-Aldrich) was added at 6 hours, and the cells were harvested at 24 hours. The cells were then pretreated with FcR-blocking Ab before being stained for 30 minutes with Cy-conjugated anti–CD4 or anti-CD8 mAb. Washed cells were fixed with 2% paraformaldehyde for 20 minutes, permeabilized with 0.3% saponin, and incubated for 40 minutes with PE-conjugated IFN-γ.
Statistical analysis
The significance of the differences between groups was analyzed using the Wilcoxon rank sum test, or the student t test. A 2-tailed P value of < .05 was considered significant. All experiments were repeated at least twice.
Results
Lymphodepletion and transfer of TNs augment antitumor immunity
TNs adoptively transferred into lymphopenic hosts expanded peripherally and acquired memory like functions.23 This homeostatic T-cell proliferation depends on self-antigen recognition and the presence of homeostatic cytokines, such as IL-7, IL-15, and IL-21.17 Previously, a study reported that the transfer of TNs after lymphodepletion augmented antitumor immunity and resulted in inhibited tumor growth.14
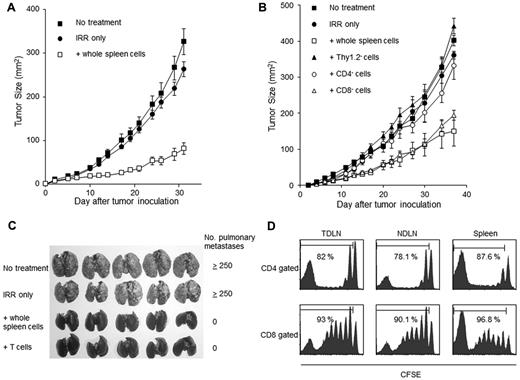
To determine whether lymphodepletion followed by the injection of TNs enhances antitumor immunity, sublethally (500 cGy) irradiated mice were intravenously injected with spleen cells (1 × 108) from naive mice and then inoculated subcutaneously with MCA205 cells (1 × 105) along the midline of the abdomen. As shown in Figure 1A, the retardation of skin tumor growth was observed in irradiated mice that were also injected with spleen cells (P < .01 vs no treatment or irradiation alone on day 31). To confirm that the transferred T cells were responsible for this inhibition of tumor progression in lymphopenic mice, we depleted Thy1.2+ cells, CD4+ cells, or CD8+ cells from donor spleen cells before transfer using magnetic beads. Sublethally irradiated mice were reconstituted with these negatively selected cells and were then challenged with 1 × 105 MCA205 cells. Depletion of Thy1.2+ cells completely abrogated the antitumor effects. The transfer of CD4+ depleted cells was associated with minimal antitumor efficacy; however, the transfer of CD8+ depleted cells resulted in antitumor effects similar to those of the transfer of unfractionated spleen cells (P < .01 for transfer of whole spleen cells or transfer of CD8+ depleted spleen cells versus irradiation alone on day 37; Figure 1B). These results indicated that the transfer of T cells, especially CD4+ T cells, was essential for the augmentation of antitumor immunity after lymphodepletion.
Lymphodepletion followed by the transfer of TNs induces TEs in TDLNs and inhibits tumor progression. (A) Mice were irradiated with 500 cGy and injected intravenously with 40 × 106 spleen cells from naive mice. These mice were then inoculated subcutaneously with 1 × 105 of MCA205 tumor cells along the midline of the abdomen. The resultant skin tumors were measured in 2 perpendicular directions 2 to 3 times per week, and the tumor areas (mm2) were recorded. (B) Sublethally irradiated lymphopenic mice were reconstituted with either Thy1.2, CD4, or CD8-depleted cell populations and then inoculated subcutaneously with MCA205 tumor cells. The depletion of Thy1.2+ or CD4+ cells, but not the depletion of CD8+ cells, abrogated the antitumor effects observed after lymphodepletion and reconstitution. (C) Sublethally irradiated mice (500cGy) were injected intravenously with either 40 × 106 whole spleen cells or with 10 × 106 magnetically isolated splenic TNs. Mice were then inoculated SD with MCA205 tumor cells to stimulate the TDLNs. Twelve days later, TDLN cells were harvested and activated in vitro with the method of CD3/IL-2 for 5 days. Ten million of activated TDLN cells were then adoptively transferred into a new group of mice with 3-day established pulmonary metastases. The TDLN cells from the mice reconstituted with whole-spleen cells and splenic TNs exhibited similar therapeutic effects. (D) Congenic Ly5.1+ spleen cells were labeled with CFSE and transferred into irradiated lymphopenic mice. These mice were then inoculated SD with 3 × 106 tumor cells to stimulate the TDLNs. Twelve days later, TDLNs, NDLNs, and spleens were harvested and analyzed for CFSE staining intensity within the Ly5.1+ subset.
Lymphodepletion followed by the transfer of TNs induces TEs in TDLNs and inhibits tumor progression. (A) Mice were irradiated with 500 cGy and injected intravenously with 40 × 106 spleen cells from naive mice. These mice were then inoculated subcutaneously with 1 × 105 of MCA205 tumor cells along the midline of the abdomen. The resultant skin tumors were measured in 2 perpendicular directions 2 to 3 times per week, and the tumor areas (mm2) were recorded. (B) Sublethally irradiated lymphopenic mice were reconstituted with either Thy1.2, CD4, or CD8-depleted cell populations and then inoculated subcutaneously with MCA205 tumor cells. The depletion of Thy1.2+ or CD4+ cells, but not the depletion of CD8+ cells, abrogated the antitumor effects observed after lymphodepletion and reconstitution. (C) Sublethally irradiated mice (500cGy) were injected intravenously with either 40 × 106 whole spleen cells or with 10 × 106 magnetically isolated splenic TNs. Mice were then inoculated SD with MCA205 tumor cells to stimulate the TDLNs. Twelve days later, TDLN cells were harvested and activated in vitro with the method of CD3/IL-2 for 5 days. Ten million of activated TDLN cells were then adoptively transferred into a new group of mice with 3-day established pulmonary metastases. The TDLN cells from the mice reconstituted with whole-spleen cells and splenic TNs exhibited similar therapeutic effects. (D) Congenic Ly5.1+ spleen cells were labeled with CFSE and transferred into irradiated lymphopenic mice. These mice were then inoculated SD with 3 × 106 tumor cells to stimulate the TDLNs. Twelve days later, TDLNs, NDLNs, and spleens were harvested and analyzed for CFSE staining intensity within the Ly5.1+ subset.
We previously demonstrated that TDLNs played a pivotal role in initiating antitumor immunity.21,24 TEs were efficiently primed in the TDLNs and had potent antitumor effects after in vitro activation. To determine whether the transfer of T cells into lymphopenic mice induced TEs in the TDLN, an experimental protocol was devised. In this model, isolated T cells (15 × 106) were transferred intravenously into irradiated mice; next, mice were inoculated SD with MCA205 cells (3 × 106) to stimulate the TDLNs. Twelve days later, inguinal TDLNs were harvested. Subsequently, the TDLN cells were activated in vitro with an immobilized anti-CD3 mAb for 2 days, and then cultured in the presence of a low dose of IL-2 (16 U/mL) for 3 days as previously described.21 The therapeutic effects of these cells when used in adoptive immunotherapy were assessed using 3-day established pulmonary MCA205 metastases. TDLN cells generated from mice reconstituted with T cells completely eliminated tumors (Figure 1C). The proliferation of the transferred T cells in lymphopenic mice was also examined. Briefly, 30 × 106 CFSE-labeled spleen cells from naive Ly5.1 mice were transfused into irradiated or nonirradiated Ly5.2 mice. Next, the Ly5.2 mice were inoculated SD with MCA205 cells. Twelve days later, the Ly5.2 mice were euthanized, and the TDLNs, the nondraining LNs (NDLNs; contralateral inguinal LNs) and the spleens were harvested. The proliferation of Ly5.1+ donor CD4+ and CD8+ T cells was assessed through CFSE dilution. Proliferation was observed for the majority of donor CD4+ and CD8+ T cells transferred into irradiated mice (Figure 1D). This proliferation seemed to be caused by homeostatic proliferation rather than tumor-antigen stimulation, because similar dilution of CFSE was observed in both TDLNs and NDLNs. In contrast, less than 20% of donor CD4+ and CD8+ T cells had divided when they were transferred into nonirradiated mice (data not shown).
Whole-body irradiation increases the percentage of CD4+CD25+Foxp3+ Tregs
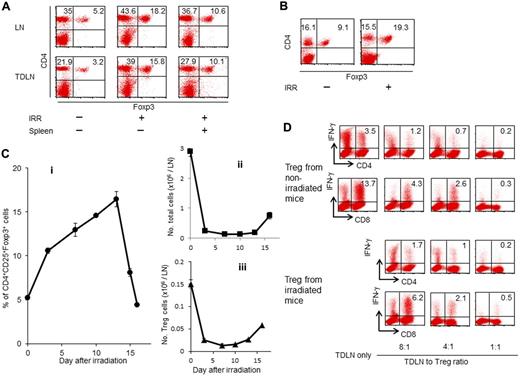
Recent evidence has shown that tumor cells induce suppressor cells to escape attack by the immune system.25 Many studies have demonstrated an increase in the frequency of Tregs was observed in cancer patients.5 Previously, we reported that the effector-to-suppressor T-cell ratio was strongly correlated with overall survival in small-cell lung cancer patients.8 Accumulating evidence suggests that Tregs may suppress antitumor immunity and contribute to tumor progression. Although lymphodepleting regimens may affect the number and functions of Tregs, the effect of lymphodepletion on Tregs is poorly understood. To investigate lymphodepletion recovery, mice were irradiated sublethally, and FACS analysis of LN cells was performed 12 days after irradiation. As demonstrated in Figure 2A, the percentage of Tregs increased from 5.2% in naive mice to 18.2% in irradiated mice. An increased percentage of Tregs in the TDLNs was also observed in irradiated animals (15.8%) compared with nonirradiated mice (3.2%). Although the transfer of naive spleen cells after irradiation reduced the percentage of Tregs in the LNs (10.6% in the NDLNs and 10.1% in the TDLNs), the percentage of Tregs was still 2- to 3-fold higher in irradiated hosts than the percentage of those in nonirradiated hosts. To determine whether irradiation increased tumor-infiltrating Tregs, irradiated mice were inoculated subcutaneously with MCA205 cells. Twelve-day skin tumors were harvested, and single-cell suspensions were analyzed using FACS. The percentage of Tregs within skin tumor tissues in irradiated mice (19.3%) was higher compared with the percentage of those cells in nonirradiated mice (9.1%; Figure 2B). To evaluate the kinetics of Tregs accumulation during recovery from lymphopenia, LNs were analyzed at different time points after irradiation. The percentage of Tregs increased as early as 3 days after irradiation (P < .0001), and re-emerged to nearly normal level by day 16 (Figure 2Ci). By contrast, irradiation significantly decreased the absolute numbers of LN cells (Figure 2Cii; P = .0003) and Tregs (Figure 2Ciii, P = .004) on day 3.
Lymphodepletion increases the percentage of CD4+CD25+Foxp3+ Tregs in LNs and tumor tissues. Irradiated mice were transferred intravenously with whole spleen cells and then inoculated with tumor cells in the right flank. Twelve days later, NDLNs (left inguinal), TDLNs (A), and tumor tissues (B) were harvested, and single-cell suspensions were prepared for FACS analysis. The percentage of CD4+CD25+Foxp3+ cells in the LNs was significantly increased in irradiated mice compared with nonirradiated mice. An elevated percentage of CD4+CD25+Foxp3+ cells was also observed in the tumor tissues of the irradiated mice. (C) Kinetics of the percentage of CD4+CD25+Foxp3+ cells (i), the absolute number of total LN cells (ii), and that of CD4+CD25+Foxp3+ cells (iii). Inguinal LNs were harvested at different time points after irradiation and analyzed by flow cytometry. (D) Normal mice were inoculated SD with 1.5 × 106 MCA205 tumor cells. Twelve-day TDLN cells were harvested and activated in vitro using the anti-CD3/IL-2 method in the absence or presence of CD4+CD25+ cells isolated from the spleens of irradiated mice or nonirradiated mice at different ratios. Activated TDLN cells were tested for antigen-specific IFN-γ production after specific stimulation as indicated.
Lymphodepletion increases the percentage of CD4+CD25+Foxp3+ Tregs in LNs and tumor tissues. Irradiated mice were transferred intravenously with whole spleen cells and then inoculated with tumor cells in the right flank. Twelve days later, NDLNs (left inguinal), TDLNs (A), and tumor tissues (B) were harvested, and single-cell suspensions were prepared for FACS analysis. The percentage of CD4+CD25+Foxp3+ cells in the LNs was significantly increased in irradiated mice compared with nonirradiated mice. An elevated percentage of CD4+CD25+Foxp3+ cells was also observed in the tumor tissues of the irradiated mice. (C) Kinetics of the percentage of CD4+CD25+Foxp3+ cells (i), the absolute number of total LN cells (ii), and that of CD4+CD25+Foxp3+ cells (iii). Inguinal LNs were harvested at different time points after irradiation and analyzed by flow cytometry. (D) Normal mice were inoculated SD with 1.5 × 106 MCA205 tumor cells. Twelve-day TDLN cells were harvested and activated in vitro using the anti-CD3/IL-2 method in the absence or presence of CD4+CD25+ cells isolated from the spleens of irradiated mice or nonirradiated mice at different ratios. Activated TDLN cells were tested for antigen-specific IFN-γ production after specific stimulation as indicated.
To evaluate the suppressive functions of Tregs increasing in irradiated mice, magnetically isolated CD4+CD25+ cells from the irradiated mice were studied. TEs were generated in the TDLNs of normal mice that were inoculated SD with MCA205 cells. These TDLN cells included tumor-specific CD4+ and CD8+ T cells.26 Twelve days after tumor-growth, the TDLN cells were harvested and stimulated in vitro with the anti-CD3/IL-2 methods in the absence or presence of CD4+CD25+ cells isolated from the irradiated or normal mice at different ratios. After further stimulation with specific MCA205 cells, the TDLN cells were analyzed for IFN-γ production. The suppressive functions of Tregs from irradiated mice were similar to those of Tregs from nonirradiated mice (Figure 2D). In addition, the phenotypic markers of the Tregs isolated from the irradiated hosts were assessed. No differences in the expression of the surface markers GITR, CTLA-4, TGF-β receptor I, TNF-α2, CD122, IL-7 receptor α, CCR7, LAG3, and folate receptor 4 were observed between Tregs from irradiated mice and Tregs from nonirradiated naive mice (data not shown).
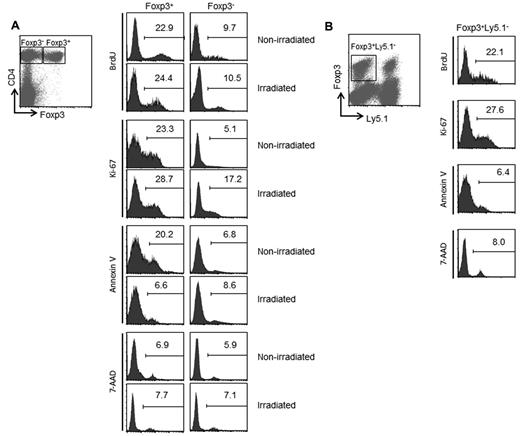
To evaluate Treg proliferation after irradiation, lymphodepleted mice received continuous administration of BrdU for 3 days. LN cells were harvested 7 days after irradiation, and were analyzed for the BrdU incorporation and the Ki-67 expression. In nonirradiated mice, 22.9% of CD4+Foxp3+ cells incorporated BrdU compared with only 9.7% of CD4+Foxp3− cells (Figure 3A). Irradiation did not affect the BrdU incorporation within CD4+Foxp3+ cells (24.4%) and CD4+Foxp3− cells (10.5%). Although irradiation increased the percentage of Ki-67+CD4+Foxp3− cells from 5.1% to 17.2%, the percentage of Ki-67+ cells was still higher in CD4+Foxp3+ Tregs than the percentage of those cells in CD4+Foxp3− cells. We next investigated whether irradiation affects the rate of cell death in Tregs. In nonirradiated mice, the percentage of annexin V+ apoptotic cells in CD4+Foxp3+ Tregs (20.2%) was higher than the percentage of those cells in CD4+Foxp3− cells (6.8%). After irradiation, the percentage of annexin V+ cells in CD4+Foxp3+ Tregs (6.6%) was reduced to the same level of % annexin V+ cells in CD4+Foxp3− cells (8.6%). By contrast, irradiation did not affect the percentage of 7-AAD+ cells within CD4+Foxp3+ and CD4+Foxp3− subsets. These results indicate that the increased percentage of Tregs during recovery from lymphopenia is because of the rapid proliferation of Tregs that survive irradiation. Our findings are consistent with previous studies that reported the rapid turnover of CD4+ Foxp3+ cells at the steady state.27,28
Analysis of proliferation and survival of Tregs during recovery from lymphopenia. (A) Irradiated or nonirradiated mice were injected intraperitoneally with 1 mg of BrdU for consecutive 3 days before sacrifice. Seven days after irradiation, single-cell suspensions were prepared from LNs and were analyzed using FACS. The percentages of BrdU+, Ki-67+, annexin V+, and 7-AAD+ cells on gated CD4+Foxp3+ and CD4+Foxp3− subsets are indicated. (B) Forty million of Ly5.1+ spleen cells were transferred into irradiated mice. LN cells from day 7 were analyzed by FACS. Histograms show the percentages of BrdU+, Ki-67+, annexin V+, and 7-AAD+ cells among Foxp3+Ly5.1− recipient cells.
Analysis of proliferation and survival of Tregs during recovery from lymphopenia. (A) Irradiated or nonirradiated mice were injected intraperitoneally with 1 mg of BrdU for consecutive 3 days before sacrifice. Seven days after irradiation, single-cell suspensions were prepared from LNs and were analyzed using FACS. The percentages of BrdU+, Ki-67+, annexin V+, and 7-AAD+ cells on gated CD4+Foxp3+ and CD4+Foxp3− subsets are indicated. (B) Forty million of Ly5.1+ spleen cells were transferred into irradiated mice. LN cells from day 7 were analyzed by FACS. Histograms show the percentages of BrdU+, Ki-67+, annexin V+, and 7-AAD+ cells among Foxp3+Ly5.1− recipient cells.
As shown in Figure 2A, transfer of naive spleen cells into irradiated lymphopenic mice reduced the percentage of Tregs. These results suggest that transfer of naive spleen cells may affect the proliferation and the survival of Tregs during recovery from lymphopenia. To address this issue, irradiated mice were reconstituted with spleen cells from Ly5.1 mice. FACS analysis of LN cells from day 7 demonstrated that transfer of naive spleen cells did not affect either the proliferation or the cell death of Ly5.1− recipient Tregs (Figure 3B). Because the transfer of naive spleen cells into irradiated mice did not decrease the absolute number of Tregs (data not shown), the reduced percentage of Tregs in irradiated reconstituted mice seemed to be because of the proliferation of donor T cells.
Treg depletion after irradiation and the transfer of naive spleen cells inhibited tumor progression
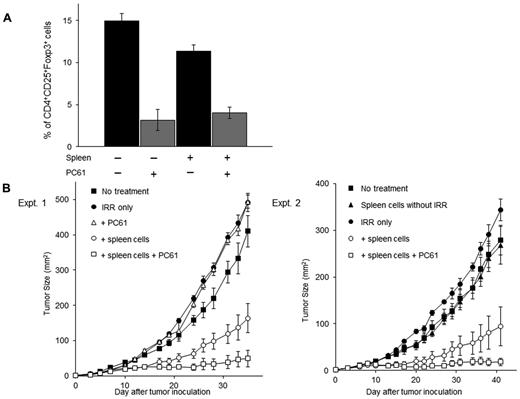
We next asked whether the inhibition of the Tregs increasing in the irradiated mice enhances antitumor immunity. In a previous study, we demonstrated that the systemic administration of anti-murine CD25 mAb PC61 suppressed Tregs and augmented the induction of TEs.29 To determine whether the administration of the anti–CD25 mAb was able to decrease the percentage of Tregs in irradiated reconstituted mice inoculated SD with MCA205 cells, these mice were also injected intraperitoneally with anti–CD25 mAb. As depicted in Figure 4A, the percentage of CD4+CD25+Foxp3+ Tregs in the TDLN cells was reduced from 14.9 ± 0.9 to 3.2 ± 1.2% in irradiated mice and from 11.3 ± 0.7 to 4.1 ± 0.7% in irradiated reconstituted mice after anti–CD25 mAb treatment. By contrast, anti–CD25 mAb treatment did not decrease the percentage of non-Tregs cells in the spleens, LNs, and bone marrow (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article).
The inhibition of Tregs augments antitumor responses in reconstituted mice. (A) Lymphodepleted mice were transferred intravenously with 40 × 106 naive spleen cells and then injected SD with MCA205 tumor cells. Next, these mice were treated with anti–CD25 mAb (PC61) to inhibit CD4+CD25+Foxp3+ Tregs. Twelve days later, the percentage of CD4+CD25+Foxp3+ Tregs in the TDLN cells were assessed by FACS analysis. Treatment with PC61 significantly reduced the percentage of CD4+CD25+Foxp3+ Tregs. (B) Reconstituted mice were inoculated subcutaneously with 1 × 105 MCA205 tumor cells. On the same day, mice were injected intraperitoneally with PC61. The combination of lymphodepletion, spleen cell transfer, and PC61 treatment significantly suppressed skin tumor growth.
The inhibition of Tregs augments antitumor responses in reconstituted mice. (A) Lymphodepleted mice were transferred intravenously with 40 × 106 naive spleen cells and then injected SD with MCA205 tumor cells. Next, these mice were treated with anti–CD25 mAb (PC61) to inhibit CD4+CD25+Foxp3+ Tregs. Twelve days later, the percentage of CD4+CD25+Foxp3+ Tregs in the TDLN cells were assessed by FACS analysis. Treatment with PC61 significantly reduced the percentage of CD4+CD25+Foxp3+ Tregs. (B) Reconstituted mice were inoculated subcutaneously with 1 × 105 MCA205 tumor cells. On the same day, mice were injected intraperitoneally with PC61. The combination of lymphodepletion, spleen cell transfer, and PC61 treatment significantly suppressed skin tumor growth.
Mice were injected intraperitoneally with anti–CD25 mAb after irradiation and reconstitution with spleen cells and were then inoculated SD with MCA205 cells along the midline of the abdomen on the same day. Two separate experiments demonstrated that anti–CD25 mAb treatment after irradiation and reconstitution significantly suppressed skin tumor growth compared with irradiation and reconstitution alone (P < .01; Figure 4B). The adoptive transfer of naive spleen cells seemed to be critical in this model system because the administration of anti–CD25 mAb to irradiated mice that were not reconstitution did not result in any antitumor effects.
Treg depletion after reconstitution enhances the induction of TEs in TDLNs
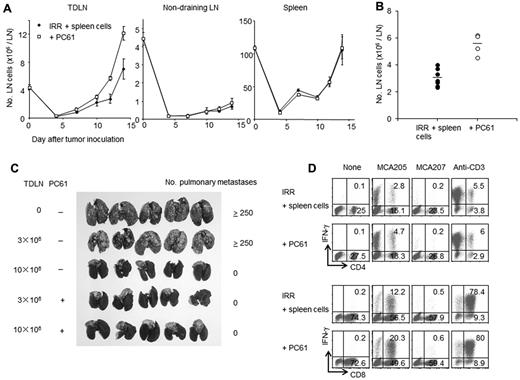
To determine whether anti–CD25 mAb treatment augments the induction of TEs, irradiated reconstituted mice were inoculated SD with MCA205 cells and then injected with anti–CD25 mAb. Figure 5A shows the number of TDLNs, NDLNs and spleen cells at different time points after tumor inoculation. Anti–CD25 mAb treatment significantly increased the number of TDLN cells (7.1 ± 1.4 × 106 versus 12.1 ± 0.9 × 106 per TDLN on day 14, P = .01165). By contrast, the numbers of NDLN cells and spleen cells were not affected by anti–CD25 mAb treatment (P = .58 for NDLN and P = .91 for spleen cells on day 14). The numbers of TDLN cells on day 12 from 7 independent experiments are presented in Figure 5B. Each dot indicates the mean number of TDLN cells from 3 to 6 mice. A significant increase of the number of TDLN cells was observed in the anti–CD25 mAb treatment group (P < .01).
Depletion of Tregs after reconstitution increases the number of TEs in the TDLNs. (A) Irradiated mice were reconstituted with 40 × 106 naive spleen cells and then inoculated SD with 3 × 106 MCA205 tumor cells in the right flank. Next, these mice were treated with PC61. The numbers of cells in the TDLNs, NDLNs, and spleens were counted on the indicated day after tumor inoculation. Treatment with PC61 significantly increased the number of TDLN cells; however, this treatment did not affect the number of NDLN cells or spleen cells. (B) Twelve-day TDLNs were harvested from mice reconstituted with normal spleen cells (40 × 106) that were with PC61 treatment or left untreated. Each dot indicates the mean number of TDLN cells from separate experiments (n = 3 to 6 mice per group). (C) Reconstituted mice were inoculated SD with MCA205 tumor cells and then treated with PC61 or left untreated. Twelve days later, TDLN cells were harvested and activated in vitro as described in “Activation of tumor-draining lymph-node cells.” Mice bearing 3-day established pulmonary metastases were treated with 3 or 10 × 106 activated TDLN cells. The TDLN cells from PC61-treated mice showed greater antitumor efficacy. (D) Twelve-day TDLN cells were harvested from reconstituted mice that were treated with PC61 or left untreated. These TDLN cells were activated in vitro with the method of CD3/IL-2 and tested for IFN-γ production after specific or nonspecific stimulation. A representative result from 3 independent experiments is shown. The further depletion of Tregs after sublethal irradiation and reconstitution increased tumor-antigen specific T cells in the TDLNs.
Depletion of Tregs after reconstitution increases the number of TEs in the TDLNs. (A) Irradiated mice were reconstituted with 40 × 106 naive spleen cells and then inoculated SD with 3 × 106 MCA205 tumor cells in the right flank. Next, these mice were treated with PC61. The numbers of cells in the TDLNs, NDLNs, and spleens were counted on the indicated day after tumor inoculation. Treatment with PC61 significantly increased the number of TDLN cells; however, this treatment did not affect the number of NDLN cells or spleen cells. (B) Twelve-day TDLNs were harvested from mice reconstituted with normal spleen cells (40 × 106) that were with PC61 treatment or left untreated. Each dot indicates the mean number of TDLN cells from separate experiments (n = 3 to 6 mice per group). (C) Reconstituted mice were inoculated SD with MCA205 tumor cells and then treated with PC61 or left untreated. Twelve days later, TDLN cells were harvested and activated in vitro as described in “Activation of tumor-draining lymph-node cells.” Mice bearing 3-day established pulmonary metastases were treated with 3 or 10 × 106 activated TDLN cells. The TDLN cells from PC61-treated mice showed greater antitumor efficacy. (D) Twelve-day TDLN cells were harvested from reconstituted mice that were treated with PC61 or left untreated. These TDLN cells were activated in vitro with the method of CD3/IL-2 and tested for IFN-γ production after specific or nonspecific stimulation. A representative result from 3 independent experiments is shown. The further depletion of Tregs after sublethal irradiation and reconstitution increased tumor-antigen specific T cells in the TDLNs.
The therapeutic effects of TDLN cells were enhanced by the inhibition of Tregs after irradiation and reconstitution. Irradiated reconstituted mice were inoculated SD with MCA205 cells and then injected with anti–CD25 mAb. After in vitro activation, the TDLN cells were evaluated for their therapeutic effects against established pulmonary metastases using adoptive immunotherapy. TDLN cells obtained from anti–CD25 mAb-treated mice had at least 3-fold higher antitumor reactivity than TDLN cells from control mice (Figure 5C).
To quantitatively analyze TEs in the TDLNs from reconstituted and Tregs-depleted mice, IFN-γ production was evaluated after stimulation with specific tumor cells. The percentages of both CD4+ and CD8+ T cells responding to specific tumor stimulation were increased in Tregs-depleted mice (Figure 5D). However, Treg depletion did not affect nonspecific TDLN-cell responses. Because the anti–CD25 treatment significantly increased the number of TDLN cells (Figure 5A-B), Treg depletion after irradiation and reconstitution increased the absolute number of TEs in the TDLN.
Radio-resistant recipient Tregs inhibit antitumor immunity during recovery from lymphopenia
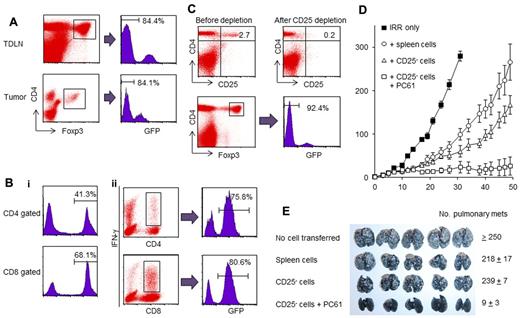
In irradiated reconstituted mice, the Treg population may consist of donor and recipient cells. To determine the origin of the Tregs after irradiation and reconstitution, irradiated mice were reconstituted with spleen cells from transgenic GFP mice and then inoculated with MCA205 cells. FACS analysis of TDLNs and tumor digests from day 12 revealed that 84.4% of the CD4+Foxp3+ Tregs cells in the TDLNs and 84.1% of the Tregs in the tumor digests were GFP− irradiated recipient cells (Figure 6A). These findings prompted us to identify the origin of the TEs in the irradiated reconstituted animals. Mice were irradiated and reconstituted with GFP+ spleen cells. TDLN cells from day 12 were harvested, activated in vitro with the method of CD3/IL-2 and then further stimulated with specific MCA205 cells. In contrast to the majority of Treg cells, which were of recipient origin, FACS analysis of freshly harvested TDLN cells demonstrated that 41.3% of CD4+ T cells and 68.1% of CD8+ T cells were derived from GFP+ donor cells (Figure 6Bi). Further, 75.8% of the CD4+ and 80.8% of the CD8+ TEs that produced IFN-γ in response to specific tumor-antigen stimulation were of donor origin (Figure 6Bii). These results suggest that the TEs originated from donor T cells, and that radio-resistant recipient Tregs interfere with the development of antitumor immunity in lymphopenic mice.
Radio-resistant recipient Tregs inhibit the development of antitumor immunity in reconstituted lymphopenic mice. (A) Forty million GFP+ spleen cells were transferred into irradiated mice that were then inoculated SD with MCA205 cells. TDLNs and tumors were harvested on day 12, and single-cell suspensions were analyzed for GFP expression within the gated CD4+Foxp3+ Tregs subset. The percentage of GFP− recipient cells among CD4+Foxp3+ cells is indicated. (B) Irradiated mice were reconstituted with GFP+ spleen cells (40 × 106) and injected SD with MCA205 tumor cells. Single-cell suspensions were prepared from the TDLNs and were analyzed using FACS. The percentage of the transferred donor cells in fresh TDLNs is indicated by the percentage of GFP+ cells (i). TDLN cells from mice reconstituted with GFP+ spleen cells were activated with the method of CD3/IL-2. Activated TDLN cells were further stimulated with MCA205 tumor digests and stained for the detection IFN-γ as described in “Intracellular IFN-γ staining.” The majority of tumor-specific cells were primed from the transferred donor GFP+ cells (ii). (C) GFP+ spleen cells were magnetically depleted of CD25+ cells. These CD25−GFP+ cells were transferred intravenously into irradiated mice. Twelve days later, LNs were harvested and stained for FACS analysis. More than 90% of the CD4+Foxp3+ cells were GFP− recipient cells. (D) Irradiated mice reconstituted with CD25− depleted spleen cells were injected with MCA205 tumor cells. These mice were then treated with anti–CD25 Abs to further inhibit the recipient Tregs. The depletion of the residual recipient-derived Tregs significantly augmented antitumor immunity. (E) TDLNs were harvested from mice reconstituted with CD25− spleen cells that were treated with anti–CD25 mAb or left untreated. Three million of activated TDLN cells were transferred into mice bearing 3-day established pulmonary metastases. The depletion of recipient Tregs enhanced the generation of TEs in the TDLNs.
Radio-resistant recipient Tregs inhibit the development of antitumor immunity in reconstituted lymphopenic mice. (A) Forty million GFP+ spleen cells were transferred into irradiated mice that were then inoculated SD with MCA205 cells. TDLNs and tumors were harvested on day 12, and single-cell suspensions were analyzed for GFP expression within the gated CD4+Foxp3+ Tregs subset. The percentage of GFP− recipient cells among CD4+Foxp3+ cells is indicated. (B) Irradiated mice were reconstituted with GFP+ spleen cells (40 × 106) and injected SD with MCA205 tumor cells. Single-cell suspensions were prepared from the TDLNs and were analyzed using FACS. The percentage of the transferred donor cells in fresh TDLNs is indicated by the percentage of GFP+ cells (i). TDLN cells from mice reconstituted with GFP+ spleen cells were activated with the method of CD3/IL-2. Activated TDLN cells were further stimulated with MCA205 tumor digests and stained for the detection IFN-γ as described in “Intracellular IFN-γ staining.” The majority of tumor-specific cells were primed from the transferred donor GFP+ cells (ii). (C) GFP+ spleen cells were magnetically depleted of CD25+ cells. These CD25−GFP+ cells were transferred intravenously into irradiated mice. Twelve days later, LNs were harvested and stained for FACS analysis. More than 90% of the CD4+Foxp3+ cells were GFP− recipient cells. (D) Irradiated mice reconstituted with CD25− depleted spleen cells were injected with MCA205 tumor cells. These mice were then treated with anti–CD25 Abs to further inhibit the recipient Tregs. The depletion of the residual recipient-derived Tregs significantly augmented antitumor immunity. (E) TDLNs were harvested from mice reconstituted with CD25− spleen cells that were treated with anti–CD25 mAb or left untreated. Three million of activated TDLN cells were transferred into mice bearing 3-day established pulmonary metastases. The depletion of recipient Tregs enhanced the generation of TEs in the TDLNs.
To confirm that Tregs from irradiated recipients suppressed antitumor immunity during recovery from lymphopenia, we depleted CD25+ Tregs cells from GFP+ donor spleen cells before the donor cells were transferred into irradiated mice (Figure 6C). FACS analysis of the TDLN cells revealed an increase in the percentage of CD4+Foxp3+ recipient Tregs after reconstitution with CD25-depleted spleen cells. To determine whether the depletion of Tregs from irradiated recipients enhances antitumor immunity, irradiated mice were reconstituted with CD25-depleted spleen cells and then inoculated with MCA205 cells. These mice were also treated with anti–CD25 mAb to deplete radio-resistant recipient Tregs. Further depletion of recipient Tregs significantly inhibited skin tumor progression compared with the depletion of donor Tregs alone (P = .001561 on day 49; Figure 6D). We next assessed whether the depletion of recipient Tregs augments the generation of TEs in the TDLN. Irradiated mice were reconstituted with CD25-depleted spleen cells, injected with MCA205 cells and then treated with anti–CD25 mAb or left untreated. After in vitro activation, TDLN cells were evaluated for their therapeutic efficacy in adoptive immunotherapy. As demonstrated by the reduced numbers of pulmonary metastases, the radio-resistant recipient Tregs suppressed the induction of TEs during reconstitution (P < .01 for transfer of CD25− cells with anti–CD25 mAb treatment versus transfer of CD25− cells without anti–CD25 mAb treatment; (Figure 6E).
Depletion of Tregs that survive cyclophosphamide treatment augments antitumor immunity
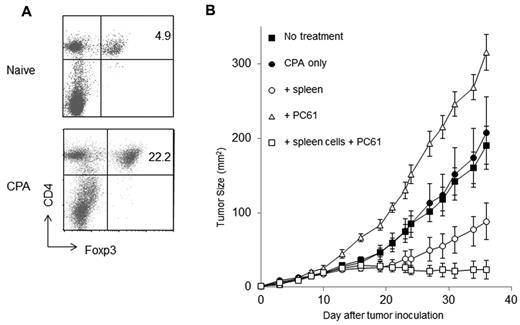
In addition to whole-body irradiation, chemotherapy has been reported to induce a lymphopenic state and augment antitumor immunity.30 We postulated that, similar to radio-resistant Tregs, residual chemo-resistant Tregs could suppress the development of antitumor immunity after chemotherapy. Cyclophosphamide has been used to enhance the antitumor effect of adoptively transferred T cells.10 To test our hypothesis, mice were injected with a sublethal dose of cyclophosphamide (5 mg per mouse). Seven days after cyclophosphamide treatment, more than 90% of lymphocytes were depleted in the treated mice (data not shown). As for irradiated mice, FACS analysis revealed a significant increase in CD4+CD25+Foxp3+ Tregs in the LNs of the cyclophosphamide-treated mice 12 days after cyclophosphamide treatment (Figure 7A). We next asked whether Treg depletion after cyclophosphamide administration augments the antitumor immunity. To avoid any direct cytotoxic effects of cyclophosphamide on tumor cells, mice were injected with 5 mg of cyclophosphamide intraperitoneally 1 day before being reconstituted with naive spleen cells and injected with tumor cells. Consistent with previous studies, the transfer of spleen cells after cyclophosphamide treatment delayed tumor progression (P = .002863 for no treatment and P = .035644 for cyclophosphamide alone; Figure 7B). Furthermore, Treg depletion with anti–CD25 mAb after cyclophosphamide treatment and reconstitution strongly inhibited skin tumor growth (P = .031039 on day 36).
The depletion of cyclophosphamide-resistant Tregs augments antitumor immunity. (A) Mice were injected intraperitoneally with cyclophosphamide (5 mg/mouse). Twelve days later, LNs were harvested for FACS analysis. The percentage of Tregs was higher after cyclophosphamide treatment. (B) Mice were treated with cyclophosphamide. One day later, mice were reconstituted with naive spleen cells and then injected with 1 × 105 MCA205 tumor cells. Anti–CD25 mAb were also administered to these mice to inhibit Tregs. Treg depletion in reconstituted cyclophosphamide-treated mice that received naive spleen cells significantly inhibited skin tumor progression.
The depletion of cyclophosphamide-resistant Tregs augments antitumor immunity. (A) Mice were injected intraperitoneally with cyclophosphamide (5 mg/mouse). Twelve days later, LNs were harvested for FACS analysis. The percentage of Tregs was higher after cyclophosphamide treatment. (B) Mice were treated with cyclophosphamide. One day later, mice were reconstituted with naive spleen cells and then injected with 1 × 105 MCA205 tumor cells. Anti–CD25 mAb were also administered to these mice to inhibit Tregs. Treg depletion in reconstituted cyclophosphamide-treated mice that received naive spleen cells significantly inhibited skin tumor progression.
Discussion
In the 1980s, lymphodepletion by irradiation or cyclophosphamide treatment was found to augment the antitumor effects of TEs.10,11 In these studies, Treg depletion was suggested to be the key mechanism underlying the effectiveness of lymphodepletion. Additional studies revealed that lymphodepletion enabled the rapid expansion of transferred TEs and enhanced effector functions.12,31 Recently, homeostatic T-cell proliferation has been thoroughly investigated in hosts with lymphopenia induced by viral infections, thymectomy or cytotoxic therapies. T-cell proliferation is driven to restore the original pool size.17 T cells that undergo homeostatic proliferation acquire memory-like phenotypes and gain several effector functions without exogenous antigenic stimulation.32,33 Extensive studies revealed that homeostatic proliferation requires T-cell receptor engagement by self-peptide/MHC complexes and exposure to common γ-chain cytokines, such as IL-7, IL-15, and IL-21.17 Gattinoni and coworkers reported that lymphodepletion failed to enhance the antitumor efficacy of TEs in IL-7 and IL-15–knockout mice.31 These authors also found that further depletion of natural killer cells from both T and B cell–deficient mice enhanced the antitumor responses of transferred TEs. Moreover, lymphodepletion through whole-body irradiation did not augment antitumor immunity in Rag-2/common γ–chain-knockout mice lacking T, B, and natural killer cells. These findings indicate that the elimination of endogenous immune cells that compete with adoptively transferred TEs for activating cytokines enhances the antitumor efficacy of transferred T cells. Importantly, Treg depletion was not the key mechanism of this enhancement because lymphodepletion augmented the antitumor efficacy of transferred TEs in Rag-1 or MHC class II-knockout mice that lack endogenous Tregs.
In this study, we demonstrated that the percentage of Tregs was increased in whole-body irradiated lymphopenic mice (Figure 2A-C). In vitro, Tregs isolated from irradiated mice exhibited suppressive function (Figure 2D). Further, the depletion of Tregs after lymphodepletion and reconstitution augmented antitumor immunity and significantly inhibited tumor progression (Figures 4B, 5). BrdU incorporation and Ki-67 expression assay demonstrated that Tregs in irradiated hosts have a higher turnover rate compared with non-Tregs counterparts (Figure 3A). These findings indicate that the Tregs that survive irradiation proliferate rapidly and suppress the development of antitumor immunity during recovery from lymphopenia. Annexin V apoptosis assay suggests the possibility that Tregs are less apoptotic during recovery from lymphopenia compared with those cells at the steady state. Further experiments are necessary to elucidate the mechanisms of the increase of Tregs after lymphodepletion in the future. Because the transfer of donor TNs was required to augment antitumor immunity in lymphopenic mice in our model, the proliferating Tregs in reconstituted mice might be of donor cell origins. To rule out this possibility, spleen cells from GFP transgenic mice were transferred into irradiated mice. FACS analysis revealed that majority of the Tregs in reconstituted mice was of recipient cell origin (Figure 6A). In contrast to the Tregs population, the majority of TEs in the TDLNs originated from donor cells (Figure 6B). The donor origin of these cells may explain why the transfer of T cells was required to induce antitumor effects in anti–CD25 mAb-treated mice (Figure 4B). To further determine whether the Tregs from irradiated mice inhibit the development of antitumor immunity during recovery from lymphopenia, donor cells were depleted of Tregs and were then transferred into irradiated recipient mice. In addition, these mice were injected with anti–CD25 mAb to deplete any residual recipient Tregs. The depletion of radio-resistant Tregs from recipients resulted in the more efficient induction of antitumor TEs in the TDLNs, and significantly inhibited tumor progression (Figure 6D-E). Thus, we concluded that radio-resistant Tregs suppressed antitumor immunity during recovery from lymphopenia.
Recently, radio-resistant Tregs have been shown to play a role in inhibiting immunity in lymphopenic hosts. Komatsu et al reported that Foxp3 mutant mice, which were deficient in Tregs, died from autoimmune disease, whereas wild-type mice that were lethally irradiated and reconstituted with BM grafts from Foxp3 mutant mice survived without evidence of autoimmunity.34 Phenotypic analyses revealed that radio-resistant recipient Tregs survived the lethal irradiation and suppressed the development of the fatal autoimmune disease. Bernard et al also demonstrated that radio-resistant Tregs could prevent the development of autoimmunity in a murine model of BM transplantation.35 Although Rag-2 knockout mice reconstituted with BM grafts from syngeneic wild-type mice developed fatal autoimmune disease, lethally irradiated immunocompetent mice reconstituted with BM from wild-type mice survived without signs of autoimmunity. The importance of the radio-resistant Tregs in this model system was demonstrated by the fact that the depletion of recipient Tregs before reconstitution reduced the survival of the reconstituted mice. Similar findings have also been reported by other investigators in different models.36,37 As previously described, T cells acquire effector phenotypes and functions during recovery from lymphopenia. Because homeostatic proliferation depends on TCR engagement with self-peptide/MHC complexes, it appears that homeostatic T-cell proliferation after lymphodepletion is potentially harmful to the host. Indeed, lymphopenia has been reported to contribute to development of autoimmune diseases.32,38 The previously discussed studies indicate that Tregs survive lymphodepletion and suppress immunity to protect hosts from auto reactive T cells during homeostatic proliferation. This study revealed that lymphodepletion increased the percentage of Tregs and that superior antitumor efficacy was achieved by further depletion of radio-resistant Tregs after sublethal irradiation. Our results provide new evidence that radio-resistant Tregs suppressed the induction of antitumor immunity during homeostatic proliferation. Moreover, we have shown, for the first time, that lymphodepletion by cyclophosphamide treatment also increased the percentage of Tregs (Figure 7A). As expected, further depletion of Tregs after cyclophosphamide treatment significantly inhibited tumor progression (Figure 7B).
Although some tumor immunotherapy studies have demonstrated survival benefits for cancer patients, the clinical effects of immunotherapies are still controversial. Our results show that the combination of lymphodepletion with the further inhibition of Tregs resistant to cytotoxic therapies represent a promising approach to enhance the efficacy of tumor immunotherapies.
There is an Inside Blood commentary on this article in this issue.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
This work was supported by KAKENHI 21590984 (S.W.).
Authorship
Contribution: J.B. performed the experiments and the analyzed data; S.W. designed the study and wrote the paper; Y.S., T.T., T.M., J.K., K.I., K.N., K.T., K.D., and C.T. performed the experiments; and S.M., H.T., J.T., H.K., H.Y., K.N., and I.N. performed the data analysis and interpretation.
Conflict-of-interest disclosure: The authors declare no competing financial interests.
Correspondence: Satoshi Watanabe, 1-754, Asahimachi-dori, Chuou-ku, Niigata-city, Niigata, Japan 951-8520; e-mail: satoshimd@yahoo.co.jp.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal