Abstract
A recombinant fusion protein linking coagulation factor IX (FIX) with human albumin (rIX-FP) has been developed to facilitate hemophilia B treatment by less frequent FIX dosing. This first-in-human dose-escalation trial in 25 previously treated subjects with hemophilia B (FIX ≤ 2 IU/dL) examined the safety and pharmacokinetics of 25, 50, and 75 IU/kg rIX-FP. Patients in the 50-IU/kg cohort underwent a comparative pharmacokinetics assessment with their previous FIX product (plasma-derived or recombinant). No allergic reactions or inhibitors were observed. Four mild, possibly treatment-related adverse events were reported. In the 50-IU/kg cohort (13 subjects), the mean half-life of rIX-FP was 92 hours, more than 5 times longer than the subjects' previous FIX product. After 25 or 50 IU/kg rIX-FP administration, the baseline-corrected mean FIX activity remained elevated at day 7 (7.4 IU/dL and 13.4 IU/dL, respectively) and day 14 (2.5 IU/dL and 5.5 IU/dL, respectively). The incremental recovery of rIX-FP was higher than both recombinant and plasma-derived FIX (1.4 vs 0.95 and 1.1 IU/dL per IU/kg, respectively). These results demonstrated both the safety and improved pharmacokinetics of rIX-FP, thus indicating this new product with extended half-life as possibly able to control and prevent bleeding with less frequent injection. The trial was registered at www.clinicaltrials.gov as no. NCT01233440.
Introduction
Hemophilia B, a deficiency of clotting factor IX (FIX), is a rare, X-linked congenital disorder that results in impaired hemostasis and manifests with frequent spontaneous bleeding episodes in joints, muscles, and soft tissues, and more rarely, life-threatening hemorrhages.1 Recurrent hemarthroses result in progressive joint damage and the development of painful and debilitating hemophilic arthropathy.2 Replacement therapy with FIX products, manufactured from plasma (pdFIX) or more recently by recombinant technology (rFIX), is presently used to control and prevent bleeding episodes.3,4 Prophylaxis is currently considered optimal care for patients with severe hemophilia5 because it reduces the incidence of all bleeding episodes and, when initiated at a very young age, also reduces the risk of arthropathy development.6 Nevertheless, currently available FIX products have a relatively short half-life of ∼ 18 hours7 ; therefore, 2 or 3 intravenous infusions per week are required to achieve effective bleeding prevention. As a life-long condition, some barriers to adherence to prescribed therapy have been identified in patients with hemophilia, including difficulties with the venous access, lack of compliance with therapy, and social and family stresses.8 In this respect, pediatric patients are particularly challenging because they should start prophylaxis early when peripheral veins are often not adequate for repeated injections. In this setting, indwelling catheters have been used to facilitate frequent dosing. However, the burden of complications associated with these devices may jeopardize the general outcome of prophylaxis and the quality of life of these children and their family members.9 Thus, a recombinant FIX product with an extended plasma half-life (t1/2) that would allow fewer injections to achieve and maintain hemostasis with either a prophylactic regimen or on-demand therapy is highly desirable.10-12
Recombinant fusion protein linking coagulation FIX with albumin (rIX-FP) is manufactured in Chinese hamster ovary cells, by genetic fusion of human recombinant albumin to the C-terminus of rFIX via a cleavable linker. The short linker peptide is derived from an endogenous FIX sequence involved in FIX activation, enabling in vivo cleavage of activated FIX from the albumin carrier moiety when required during physiologic blood coagulation.13 Albumin has been selected as the ideal fusion partner because it is the most abundant plasma protein and is a natural carrier molecule that is inherently inert to the immune system. Furthermore, albumin has a very long half-life of ∼ 20 days, and its metabolism is well known. Albumin has also been widely used as a protein stabilizer in commercial pharmaceuticals.14 Moreover, recombinant albumin has been successfully fused to several therapeutic proteins, including insulin, hirudin, and interferon.15-17 Thus, the albumin fusion technology for extending the half-life of FIX takes advantage of the biologic characteristics of albumin while at the same time allows physiologic function of an unchanged wild-type FIX.
In animal studies, rIX-FP in both rats and monkeys was well tolerated with no findings suggesting local, systemic toxicity, or thrombogenic risk at doses up to 500 IU/kg. Intravenous administration of rIX-FP to FIX-deficient mice revealed a significant dose-dependent reduction of total blood loss, time to hemostasis, and activated partial thromboplastin time.13
We report the results of the first-in-human dose-escalation study carried out in previously treated subjects with hemophilia B to assess the safety of rIX-FP. In addition, the pharmacokinetics (PK) of this new product was extensively evaluated in comparison with the PK properties of the subjects' previously used FIX products.
Methods
Study subjects
The criteria for subject selection were based on the draft “Guideline on the Clinical Investigation of Recombinant and Human Plasma-Derived Factor IX Products” by the Committee for medicinal products for human use.18 Patients were previously treated (≥ 150 exposure days to FIX products) males with hemophilia B (FIX activity ≤ 2 IU/dL) and ages 12-65 years. Patients with a history of neutralizing antibodies (inhibitors) to FIX, a CD4+ lymphocyte count < 200/mm3 or coagulation disorders other than hemophilia B were excluded from participation. Patients were recruited from 16 sites in 6 countries (Austria, France, Germany, Israel, Italy, and Spain). All subjects provided written informed consent. The study was approved by independent ethics committees of all participating centers and was conducted in accordance with good clinical practice and the Declaration of Helsinki. The trial was registered at www.clinicaltrials.gov as no. NCT01233440.
Trial design
The trial was a first-in-human prospective, multicenter, open-label, dose-escalation study to evaluate the safety and PK of 25, 50, and 75 IU/kg rIX-FP in subjects with hemophilia B. Twenty-five subjects were enrolled to ensure at least 13 evaluable subjects in the 50-IU/kg dosing group, and at least 4 evaluable subjects in both the 25- and 75-IU/kg rIX-FP dosing groups. All subjects received rIX-FP in a nonbleeding state and after a washout period of at least 4 days from their last dose of the previous FIX product. If a subject experienced a non–life-threatening hemorrhage after screening, he had to be treated with his previous product, and then rIX-FP infused at least 2 weeks after the bleeding episode resolved. Dosing of the next dose cohort commenced after review of the day 28 safety data from the first 4 subjects by the Data Review Committee. Patients were permitted to participate in up to 2 rIX-FP dosing cohorts on a voluntary basis, independently on the results of the first arm and with a wash-out period of at least 14 days between rIX-FP doses. Inhibitor testing at day 28 was performed at least 4 days after the last dose of his previous FIX product.
Patients in the 50-IU/kg cohort also received a single dose of their previously used commercially available product, either pdFIX or rFIX, for PK assessment. The subjects received 50 IU/kg of previous FIX product either before or after rIX-FP administration, and a washout period of at least 4 days after the infusion of the previous FIX product or at least 14 days after the rIX-FP infusion was required.
All bleeding events that occurred during the study were treated with the subjects' previous FIX product. All subjects on prophylaxis at study entry received their previous FIX products according to the same dosing regimen in the period between completion of PK assessment and end-of-study visit (≥ 28 days after the last rIX-FP administration).
Trial objectives and endpoints
The primary objective of the study was to assess the safety of intravenous administration of rIX-FP. Safety was determined by evaluating the frequency of adverse events, occurrence of inhibitors against FIX, antibody development against rIX-FP, local tolerability, physical examination, vital signs, urinalysis, and laboratory changes (hematology, biochemistry, D-dimer, prothrombin fragment 1 + 2 [F1 + 2], thrombin-antithrombin complex) over time.
The secondary objective of the study was to evaluate the PK parameters after a single intravenous dose of 50 IU/kg rIX-FP. Additional objectives of the study were to evaluate the PK parameters of rIX-FP at 25 and 75 IU/kg and compare PK parameters of rIX-FP with the previous FIX product (either pdFIX or rFIX) at 50 IU/kg. PK parameters were based on FIX activity and FIX antigen levels; these were baseline-corrected using the predose concentration of FIX activity and antigen, respectively.
Blood samples for measurement of FIX activity and antigen were collected before dosing rIX-FP and at 30 minutes, 3, 6, 24, 48, 72, 120, 168 (day 7), 240 and 336 hours (day 14) after infusion. Blood samples for analysis of FIX activity of previous FIX products were collected before dosing and at 30 minutes, 1-3, 8-12, 20-26, and 32-48 hours after infusion. All PK parameters were calculated using the actual collection times, according to International Society for Thrombosis and Haemostasis recommendations.19,20
Analytical methods
FIX activity was measured using a validated 1-stage clotting method. Briefly, the test samples were mixed with equal amounts of FIX-depleted plasma and tested by in vitro determination of activated partial thromboplastin time using Pathromtin SL (Siemens Healthcare Diagnostics) as activator reagent, rIX-FP activity determination was performed using the Behring Coagulation System. The results were interpreted using a reference curve, which was prepared from standard human plasma calibrated by the manufacturer against WHO standard (International Blood Coagulation Factors II, VII, IX, X human plasma) for FIX, and the results are reported in international units per decaliter of norm or international units. rIX-FP and previous FIX product activity was measured with the same assay in accordance with the World Health Plasma FIX standard (99/826) to facilitate comparison of PK parameters. Therefore, 1 IU of rFIX or pdFIX is equivalent to 1 IU of rIX-FP. In addition, FIX antigen was measured using a validated ELISA assay (Affinity Biologicals).
Inhibitors were titrated by the Bethesda method according to the Nijmegen modification, a coagulation assay based on in vitro determination of activated partial thromboplastin time in human citrated plasma. A result ≥ 0.6 Bethesda units was defined as a positive result.
A tiered approach to immunogenicity testing for rIX-FP was used during the study. Antibodies to rIX-FP were tested in all subjects before rIX-FP exposure and 4 weeks after exposure. A direct binding ELISA assay was used to detect antibodies against rIX-FP; if a positive signal was obtained, the plasma sample was retested in a separate direct binding ELISA assay to confirm the specific antibody signal and to discriminate between antibodies against plasma-derived FIX, recombinant FIX (BeneFIX) and albumin.
The analyses of FIX activity, FIX antigen, inhibitors, and antibodies against rIX-FP were performed in the central laboratory at CSL Behring.
Pharmacokinetic analysis and statistical methods
The number of subjects included in the analyses was not based on a formal statistical assessment but aimed at providing descriptive data on the safety and PK profile of rIX-FP in accordance with the recommendations in the CHMP Guideline on the clinical investigation of recombinant and human plasma-derived FIX products (July 2009). Subjects were included in the PK analysis if they had more than 1 postdose PK sample drawn with no sample quality issues as reported by the central laboratory and who did not receive any FIX product for the treatment of a bleed during the PK sampling period. The PK analysis was performed by standard noncompartmental analysis for both rIX-FP and previous FIX products using WinNonlin software (Pharsight). PK parameters included: area under the curve to the last sample with quantifiable drug concentration (AUC0-t); area under the curve from the time of dosing extrapolated to infinity, based on the last observed FIX concentration (AUC0-inf); maximum observed FIX concentration (Cmax); incremental recovery (IR0–30 minutes) according to the formula C30 minutes (IU/dL)/dose (IU/kg); terminal half life (t1/2); total body clearance, normalized to body weight (CL); volume of distribution based on the t1/2, normalized to body weight (Vz); volume of distribution at steady state, normalized to body weight (Vss); and mean residence time extrapolated to infinity (MRT). PK parameters were referred to the declared product potency.
Results were summarized by dose cohort, and PK analysis of the previous FIX products summarized separately by product type (rFIX and pdFIX).
Dose proportionality of several PK parameters, including AUC0-t, AUC0-inf, and Cmax, were assessed over 25, 50, and 75 IU/kg rIX-FP by the power model. In this mixed model, the repeated measure of subject was included as a random effect to account for subjects who participated in more than 1 dose cohort. Assuming a linear relationship between the natural log (ln)–transformed parameter and ln(dose) term, the estimate of the slope (along with a 95% confidence interval [CI]) was used to determine the degree of nonproportionality. If the 95% CI for the slope contained 1, then dose proportionality was confirmed.
Post hoc analyses were performed to compare the treatment difference between rIX-FP and the previous FIX products for selected parameters. Analyses of log-transformed PK parameters were performed using a mixed model with subject as a random effect and treatment (rIX-FP or previous FIX), type of previous FIX product (pdFIX or rFIX), and treatment by type of previous FIX treatment interaction as independent variables. Least squares means estimates and 95% CIs were back-transformed to the original scale to provide ratios of rIX-FP to pdFIX and rFIX, and the 95% CIs about these ratios.
The safety end points were summarized using descriptive statistics, including all subjects exposed to rIX-FP (safety population).
Drug product
rIX-FP is a single-chain glycoprotein with a molecular weight of ∼ 125 000 Da, synthesized in Chinese hamster ovary cells. The manufacturing and formulation do not include the addition of excipients from animal or human origin.13 rIX-FP is a highly purified recombinant fusion protein linking recombinant human coagulation FIX with recombinant human albumin by a short, cleavable linker derived from an endogenous FIX sequence involved in FIX activation. The linker is cleaved from the fusion protein by the same enzymes, such as coagulation factor XIa or factor VIIa/tissue factor, which activate FIX during the process of blood coagulation, removing the albumin moiety. rIX-FP was supplied as a lyophilized sterile formulation intended for intravenous injection in single-use vials of 500 and 1000 IU/vial, and was reconstituted with 2.5 mL sterile water for injection.
Results
Study subjects
A total of 26 subjects were screened. Twenty-five subjects were enrolled, and 1 subject was withdrawn from the study before rIX-FP dosing because of screening failure (severe bleeding event). All subjects completed the study 28 days after the last rIX-FP infusion. Patient demographics were similar across dose groups; the subject population was predominantly white (96%) with a broad age range from 15 to 58 years (mean, 35 years; median, 31 years). One subject was younger than 18 years. Table 1 summarizes subject participation in the PK studies according to rIX-FP dose and prior FIX product type. Seven subjects were included in 2 rIX-FP cohorts; 3 received 25 and 50 IU/kg rIX-FP, 1 received 25 and 75 IU/kg rIX-FP, and 3 received 50 and 75 IU/kg rIX-FP. In addition, 15 subjects received a single dose of 50 IU/kg of their previous FIX product, either pdFIX (n = 5) or rFIX (BeneFIX, n = 10).
Patient disposition
| . | rIX-FP . | pdFIX or rFIX . | Total n (%) . | ||
|---|---|---|---|---|---|
| 25 IU/kg n (%) . | 50 IU/kg n (%) . | 75 IU/kg n (%) . | 50 IU/kg n (%) . | ||
| Patients enrolled | 9 | 14 | 9 | 15 | 25 |
| Analysis populations | |||||
| Safety population | 9 (100.0) | 14 (100.0) | 9 (100.0) | 15 (100.0) | 25 (100.0) |
| PK population | 7 (77.8) | 13 (92.9) | 8 (88.9) | 12 (80.0) | 22 (88.0) |
| Completed study | 9 (100.0) | 14 (100.0) | 9 (100.0) | 15 (100.0) | 25 (100.0) |
| . | rIX-FP . | pdFIX or rFIX . | Total n (%) . | ||
|---|---|---|---|---|---|
| 25 IU/kg n (%) . | 50 IU/kg n (%) . | 75 IU/kg n (%) . | 50 IU/kg n (%) . | ||
| Patients enrolled | 9 | 14 | 9 | 15 | 25 |
| Analysis populations | |||||
| Safety population | 9 (100.0) | 14 (100.0) | 9 (100.0) | 15 (100.0) | 25 (100.0) |
| PK population | 7 (77.8) | 13 (92.9) | 8 (88.9) | 12 (80.0) | 22 (88.0) |
| Completed study | 9 (100.0) | 14 (100.0) | 9 (100.0) | 15 (100.0) | 25 (100.0) |
Patients were permitted to participate in up to 2 rIX-FP dosing cohorts, and a wash-out period of at least 14 days between doses was required. Seven subjects participated in more than 1 arm as follows: 3 subjects participated in the 25-IU/kg and 50-IU/kg arms, 3 subjects participated in the 50-IU/kg and 75-IU/kg arms, and 1 subject participated in the 25-IU/kg and 75-IU/kg arms. The safety population included all subjects exposed to rIX-FP. Subjects were included in the PK analysis if they had more than 1 postdose PK sample drawn with no sample quality issues as reported by the central laboratory and who did not receive any FIX product for the treatment of a bleed during the PK sampling period.
Safety
rIX-FP was well tolerated in all subjects. There were no hypersensitivity reactions. None of the subjects developed inhibitors to FIX or antibodies to rIX-FP after rIX-FP administration. When the other safety-related parameters were assessed before and after rIX-FP exposure, no unexpected findings, no dose-related trends in markers of activation of coagulation (thrombin-antithrombin complex, F1 + 2 and D-dimer), and no clinical signs or symptoms of thrombosis were observed.
Of the 25 subjects exposed to rIX-FP, 13 (52%) reported 22 treatment-emergent adverse events after rIX-FP administration. All adverse events were reported as mild in severity except one (abdominal pain), which was considered moderate and unrelated to rIX-FP treatment. Four adverse events (mild headache, feeling hot 50 minutes after injection, mild constipation, and mild erythema at the injection site), which resolved on the same day without treatment, were reported in 3 subjects and considered to be possibly related to rIX-FP treatment.
There were a total of 18 bleeding events in 12 subjects (48%) during the study. Of these, 9 spontaneous hemorrhages were reported in 6 subjects: 2 occurred in 1 subject on day 14 (mouth bleed) and day 15 (joint bleed) after a single dose of 25 IU/kg rIX-FP, after FIX activity had returned to baseline levels. The remaining 7 spontaneous hemorrhages occurred either during the screening period (n = 3) or during the safety follow-up period between completion of PK assessment after administration of previous FIX product and day 28 (n = 4). No spontaneous hemorrhages occurred during the 14-day PK period in subjects receiving 50 or 75 IU/kg rIX-FP.
Pharmacokinetics
Fourteen subjects participated in the PK assessment of 50 IU/kg rIX-FP and 50 IU/kg of their previous FIX products. One subject participated in the PK of 50 IU/kg of his previous FIX product and 75 IU/kg of rIX-FP. Overall, 18 subjects participated in PK evaluation of either 25 or 75 IU/kg rIX-FP (Table 1). Four subjects were not included in the rIX-FP PK population because of either requiring on-demand treatment with FIX (n = 2) or errors in the collection of sample dates/times that could not be reconciled (n = 1), or issues with sample quality (n = 1).
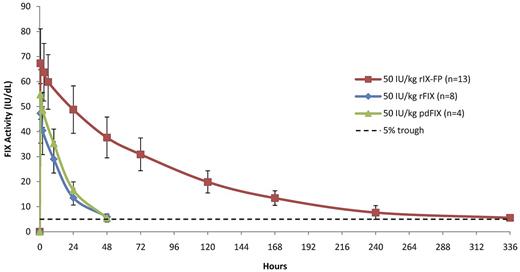
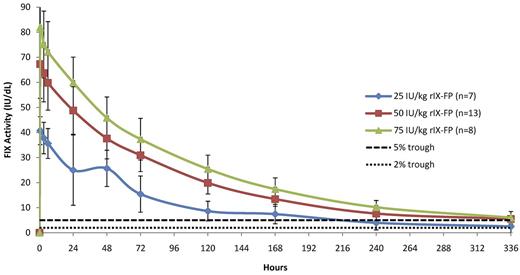
The baseline-corrected FIX activity and antigen concentrations measured in the PK population at selected time points after 25 IU/kg, 50 IU/kg, and 75 IU/kg of rIX-FP and 50 IU/kg of rFIX and pdFIX are reported in Table 2. The mean FIX concentration was higher than the baseline level in all rIX-FP dose cohorts at the end of the sampling period (after 14 days). At this final time point (336 hours), the baseline-corrected FIX activity was ∼ 5 IU/dL after a dose of 50 IU/kg rIX-FP, similar to the FIX trough level measured 48 hours after 50 IU/kg of either rFIX or pdFIX (Table 2). Figure 1 shows the mean baseline-corrected FIX activity concentrations versus time of 50 IU/kg rIX-FP, rFIX, and pdFIX. The mean baseline-corrected FIX activity concentrations versus time of 25, 50, and 75 IU/kg rIX-FP are reported in Figure 2, demonstrating that FIX trough levels > 5 IU/dL were maintained at 168 hours (day 7) and a FIX activity of 2 IU/dL was still measurable at 336 hours (day 14) in the lowest dose cohort.
Baseline-corrected FIX activity and antigen in plasma after a single dose of rIX-FP and previous FIX product
| . | rIX-FP . | Previous FIX . | |||
|---|---|---|---|---|---|
| 25 IU/kg (n = 7) . | 50 IU/kg (n = 13) . | 75 IU/kg (n = 8) . | rFIX: 50 IU/kg (n = 8) . | pdFIX: 50 IU/kg (n = 4) . | |
| 30 min | |||||
| FIX activity | 40.70 (5.58) | 67.31 (13.72) | 81.91 (14.20) | 47.26 (11.90) | 54.80 (9.89) |
| FIX antigen | 36.93 (7.66) | 69.15 (34.59) | 99.23 (22.53) | 35.68 (22.69) | 53.62 (40.58) |
| 48 h | |||||
| FIX activity | 25.67 (7.24) | 37.62 (8.17) | 45.81 (8.34) | 5.96 (1.07) | 5.45 (1.61) |
| FIX antigen | 29.40 (15.76) | 25.63 (17.00) | 44.54 (12.30) | 3.41 (3.08) | 7.35 (9.11) |
| 168 h | |||||
| FIX activity | 7.41 (3.87) | 13.41 (2.91) | 17.39 (4.46) | — | — |
| FIX antigen | 8.98 (4.49) | 9.56 (5.73) | 14.02 (7.26) | — | — |
| 336 h | |||||
| FIX activity | 2.50 (2.66) | 5.54 (2.00) | 6.01 (2.45) | — | — |
| FIX antigen | 2.63 (2.29) | 4.61 (2.96) | 7.80 (4.41) | — | — |
| . | rIX-FP . | Previous FIX . | |||
|---|---|---|---|---|---|
| 25 IU/kg (n = 7) . | 50 IU/kg (n = 13) . | 75 IU/kg (n = 8) . | rFIX: 50 IU/kg (n = 8) . | pdFIX: 50 IU/kg (n = 4) . | |
| 30 min | |||||
| FIX activity | 40.70 (5.58) | 67.31 (13.72) | 81.91 (14.20) | 47.26 (11.90) | 54.80 (9.89) |
| FIX antigen | 36.93 (7.66) | 69.15 (34.59) | 99.23 (22.53) | 35.68 (22.69) | 53.62 (40.58) |
| 48 h | |||||
| FIX activity | 25.67 (7.24) | 37.62 (8.17) | 45.81 (8.34) | 5.96 (1.07) | 5.45 (1.61) |
| FIX antigen | 29.40 (15.76) | 25.63 (17.00) | 44.54 (12.30) | 3.41 (3.08) | 7.35 (9.11) |
| 168 h | |||||
| FIX activity | 7.41 (3.87) | 13.41 (2.91) | 17.39 (4.46) | — | — |
| FIX antigen | 8.98 (4.49) | 9.56 (5.73) | 14.02 (7.26) | — | — |
| 336 h | |||||
| FIX activity | 2.50 (2.66) | 5.54 (2.00) | 6.01 (2.45) | — | — |
| FIX antigen | 2.63 (2.29) | 4.61 (2.96) | 7.80 (4.41) | — | — |
— indicates not applicable.
Mean (SD) plasma FIX activity levels and FIX antigen levels at defined time points after a single dose of rIX-FP. Mean plasma FIX activity levels and FIX antigen levels at defined time points are also presented for previous FIX products (50 IU/kg rFIX or pdFIX).
Linear plot of baseline-corrected FIX activity level after the infusion of 50 IU/kg of rIX-FP and previous FIX product (PK population). Mean FIX activity levels of 50 IU/kg rIX-FP, 50 IU/kg rFIX, and 50 IU/kg pdFIX were measured in international units per decaliter. Vertical bars represent SD. A horizontal dotted line represents the 5-IU/dL FIX activity level.
Linear plot of baseline-corrected FIX activity level after the infusion of 50 IU/kg of rIX-FP and previous FIX product (PK population). Mean FIX activity levels of 50 IU/kg rIX-FP, 50 IU/kg rFIX, and 50 IU/kg pdFIX were measured in international units per decaliter. Vertical bars represent SD. A horizontal dotted line represents the 5-IU/dL FIX activity level.
Linear plot of baseline-corrected FIX activity level after infusion of 25, 50, or 75 IU/kg of rIX-FP (PK population). Mean FIX activity levels of 25, 50, and 75 IU/kg rIX-FP were measured in international units per decaliter. Vertical bars represent SD. A horizontal dotted line represents the 2-IU/dL and 5-IU/dL FIX activity levels.
Linear plot of baseline-corrected FIX activity level after infusion of 25, 50, or 75 IU/kg of rIX-FP (PK population). Mean FIX activity levels of 25, 50, and 75 IU/kg rIX-FP were measured in international units per decaliter. Vertical bars represent SD. A horizontal dotted line represents the 2-IU/dL and 5-IU/dL FIX activity levels.
PK parameters for rIX-FP in the 3 dose cohorts and previous FIX products at 50 IU/kg were evaluated by noncompartmental analysis, and the results are summarized in Table 3. The albumin fusion technology extended the half-life of FIX, with a single dose of 50 IU/kg rIX-FP exhibiting a mean t1/2 of ∼ 92 hours and a slow CL of 0.75 mL/hr per kg.
PK parameters after a single dose of rIX-FP and previous FIX product according to noncompartmental analysis
| Parameter (unit) baseline-corrected . | rIX-FP . | Previous FIX . | |||
|---|---|---|---|---|---|
| 25 IU/kg (n = 7) . | 50 IU/kg (n = 13) . | 75 IU/kg (n = 8) . | rFIX (n = 8) . | pdFIX (n = 4) . | |
| AUC0-inf, h × IU/dL | |||||
| Mean | 4192.42 | 7089.87 | 8995.24 | 976.76 | 1086.55 |
| SD | 1627.90 | 1622.83 | 1757.80 | 164.82 | 207.65 |
| Cmax, IU/dL | |||||
| Mean | 41.14 | 69.28 | 82.04 | 47.26 | 54.80 |
| SD | 5.33 | 13.00 | 14.27 | 11.90 | 9.89 |
| IR, IU/dL per IU/kg | |||||
| Mean | 1.653 | 1.376 | 1.084 | 0.945 | 1.096 |
| SD | 0.19 | 0.28 | 0.19 | 0.24 | 0.20 |
| Half-life, h | |||||
| Mean | 104.71 | 91.57 | 98.82 | 17.23 | 14.59 |
| SD | 55.08 | 20.74 | 17.48 | 2.28 | 1.73 |
| CL, mL/h per kg | |||||
| Mean | 0.73 | 0.75 | 0.87 | 5.24 | 4.76 |
| SD | 0.46 | 0.19 | 0.17 | 0.85 | 1.08 |
| Vz, mL/kg | |||||
| Mean | 91.6 | 95.0 | 123.1 | 130.6 | 98.7 |
| SD | 43.5 | 20.3 | 28.3 | 29.9 | 14.9 |
| Vss, mL/kg | |||||
| Mean | 85.4 | 91.6 | 119.4 | 132.5 | 99.1 |
| SD | 24.2 | 15.0 | 27.0 | 34.1 | 15.5 |
| MRT, h | |||||
| Mean | 136.00 | 127.03 | 138.13 | 25.13 | 21.13 |
| SD | 47.27 | 22.66 | 20.69 | 3.72 | 2.49 |
| Parameter (unit) baseline-corrected . | rIX-FP . | Previous FIX . | |||
|---|---|---|---|---|---|
| 25 IU/kg (n = 7) . | 50 IU/kg (n = 13) . | 75 IU/kg (n = 8) . | rFIX (n = 8) . | pdFIX (n = 4) . | |
| AUC0-inf, h × IU/dL | |||||
| Mean | 4192.42 | 7089.87 | 8995.24 | 976.76 | 1086.55 |
| SD | 1627.90 | 1622.83 | 1757.80 | 164.82 | 207.65 |
| Cmax, IU/dL | |||||
| Mean | 41.14 | 69.28 | 82.04 | 47.26 | 54.80 |
| SD | 5.33 | 13.00 | 14.27 | 11.90 | 9.89 |
| IR, IU/dL per IU/kg | |||||
| Mean | 1.653 | 1.376 | 1.084 | 0.945 | 1.096 |
| SD | 0.19 | 0.28 | 0.19 | 0.24 | 0.20 |
| Half-life, h | |||||
| Mean | 104.71 | 91.57 | 98.82 | 17.23 | 14.59 |
| SD | 55.08 | 20.74 | 17.48 | 2.28 | 1.73 |
| CL, mL/h per kg | |||||
| Mean | 0.73 | 0.75 | 0.87 | 5.24 | 4.76 |
| SD | 0.46 | 0.19 | 0.17 | 0.85 | 1.08 |
| Vz, mL/kg | |||||
| Mean | 91.6 | 95.0 | 123.1 | 130.6 | 98.7 |
| SD | 43.5 | 20.3 | 28.3 | 29.9 | 14.9 |
| Vss, mL/kg | |||||
| Mean | 85.4 | 91.6 | 119.4 | 132.5 | 99.1 |
| SD | 24.2 | 15.0 | 27.0 | 34.1 | 15.5 |
| MRT, h | |||||
| Mean | 136.00 | 127.03 | 138.13 | 25.13 | 21.13 |
| SD | 47.27 | 22.66 | 20.69 | 3.72 | 2.49 |
n indicates number of subjects; AUC0-inf, area under the curve from the time of dosing extrapolated to infinity, based on the last observed FIX concentration; Cmax, maximum observed FIX concentration; IR, incremental recovery according to the formula C30 minutes (IU/dL)/dose (IU/kg); CL, plasma clearance; Vz, volume of distribution based on the t1/2, normalized to body weight; Vss, volume of distribution at steady state, normalized to body weight; and MRT, mean residence time extrapolated to infinity.
PK parameters for rIX-FP were compared with the previous FIX products (pdFIX and rFIX) in the subset of subjects receiving both 50 IU/kg rIX-FP and previous FIX products (Table 4). There was a significant difference in the mean IR0-30 minutes, t1/2, AUC0-inf, CL, MRT, and Vz of rIX-FP compared with recombinant FIX. In addition, the mean IR0-30 minutes, t1/2, AUC0-inf, CL, and MRT were significantly different compared with plasma-derived FIX. The mean incremental recovery of rIX-FP was 29% higher compared with pdFIX and 44% higher compared with rFIX. The mean t1/2 of rIX-FP was 5.8 or 5.3 times longer compared with the pdFIX or rFIX product, respectively. There was an ∼ 7-fold difference in mean AUC and CL of rIX-FP compared with rFIX and an ∼ 6-fold difference compared with pdFIX. The Vz of rIX-FP was ∼ 28% lower compared with rFIX but similar to pdFIX.
Comparison of baseline-adjusted PK parameters based on FIX activity in 50 IU/kg rIX-FP, pdFIX, and rFIX
| PK parameter . | rIX-FP (n = 8) . | rFIX (n = 8) . | rIX-FP/ rFIX ratio . | 95% lower CI . | 95% upper CI . | P . | rIX-FP (n = 4) . | pdFIX (n = 4) . | rIX-FP/pdFIX ratio . | 95% lower CI . | 95% upper CI . | P . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AUC0-inf h × IU/dL | 7142 | 965 | 7.40 | 6.27 | 8.74 | < .0001 | 6661 | 1070 | 6.23 | 4.93 | 7.87 | < .0001 |
| IR, IU/dL per IU/kg | 1.32 | 0.92 | 1.44 | 1.25 | 1.65 | .0002 | 1.40 | 1.08 | 1.29 | 1.06 | 1.57 | .0145 |
| Half-life, h | 89.88 | 17.10 | 5.26 | 4.20 | 6.59 | < .0001 | 84.73 | 14.51 | 5.84 | 4.25 | 8.03 | < .0001 |
| CL, mL/h per kg | 0.71 | 5.18 | 0.14 | 0.12 | 0.16 | < .0001 | 0.74 | 4.67 | 0.16 | 0.12 | 0.20 | < .0001 |
| MRT, h | 128.87 | 24.90 | 5.17 | 4.29 | 6.24 | < .0001 | 116.83 | 21.01 | 5.56 | 4.26 | 7.25 | < .0001 |
| Vz, mL/kg | 0.92 | 1.28 | 0.72 | 0.59 | 0.87 | .0037 | 0.90 | 0.98 | 0.92 | 0.70 | 1.21 | .513 |
| PK parameter . | rIX-FP (n = 8) . | rFIX (n = 8) . | rIX-FP/ rFIX ratio . | 95% lower CI . | 95% upper CI . | P . | rIX-FP (n = 4) . | pdFIX (n = 4) . | rIX-FP/pdFIX ratio . | 95% lower CI . | 95% upper CI . | P . |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| AUC0-inf h × IU/dL | 7142 | 965 | 7.40 | 6.27 | 8.74 | < .0001 | 6661 | 1070 | 6.23 | 4.93 | 7.87 | < .0001 |
| IR, IU/dL per IU/kg | 1.32 | 0.92 | 1.44 | 1.25 | 1.65 | .0002 | 1.40 | 1.08 | 1.29 | 1.06 | 1.57 | .0145 |
| Half-life, h | 89.88 | 17.10 | 5.26 | 4.20 | 6.59 | < .0001 | 84.73 | 14.51 | 5.84 | 4.25 | 8.03 | < .0001 |
| CL, mL/h per kg | 0.71 | 5.18 | 0.14 | 0.12 | 0.16 | < .0001 | 0.74 | 4.67 | 0.16 | 0.12 | 0.20 | < .0001 |
| MRT, h | 128.87 | 24.90 | 5.17 | 4.29 | 6.24 | < .0001 | 116.83 | 21.01 | 5.56 | 4.26 | 7.25 | < .0001 |
| Vz, mL/kg | 0.92 | 1.28 | 0.72 | 0.59 | 0.87 | .0037 | 0.90 | 0.98 | 0.92 | 0.70 | 1.21 | .513 |
The estimated means, ratio of treatment differences, and 95% CI about the ratios are presented for rIX-FP compared with rFIX and to pdFIX as matched pairs. One subject from rIX-FP 50-IU/kg PK population was excluded from the analysis because of the lack of evaluable PK results for the previous FIX product resulting from the occurrence of a bleeding event.
The dose proportionality of several baseline-corrected PK parameters, including AUC0-t, AUC0-inf, and Cmax were assessed over 25, 50, and 75 IU/kg rIX-FP by visual examination, which showed an approximately dose-proportional increase. Exploratory statistical analyses were performed using the power model to further characterize this relationship. An approximately dose-proportional increase in AUC0-t over the 3 doses was observed, with an estimated slope of 0.86 (95% CI, 0.679-1.041) based on FIX activity. For AUC0-inf and Cmax, the results indicated estimated slope of 0.71 (95% CI, 0.607-0.803) and 0.65 (95% CI, 0.482-0.818), respectively, based on FIX activity. All parameters calculated from FIX antigen levels were dose proportional over the dose range, with an estimated slope (β) in the power model of 0.959, 0.901, and 0.811 for AUC0-t, AUC0-inf, and Cmax, respectively.
Discussion
Prophylactic regimens currently used to prevent bleeding in severe hemophilia B patients consist of intravenous infusions of FIX products 2 or 3 times per week; this treatment modality usually improves the quality of life of patients. However, the implementation as well as long-term maintenance of such a treatment regimen, because of the relatively short half-life of commercially available FIX products, are often demanding for patients and their families. The leading reasons for poor adherence to the prescribed prophylactic treatment regimen or skipping the administration are forgetfulness, lack of time for treatment, and convenience, particularly in patients on high-intensity regimens.8 A recombinant FIX product with a prolonged half-life would have the potential to dose less frequently, to prevent the occurrence of breakthrough bleeding with a less frequent prophylaxis regimen, and to provide effective on-demand treatment for patients with this life-long, debilitating bleeding disorder. To improve adherence to treatment and decrease the burden on patients, a recombinant fusion protein linking coagulation FIX with human albumin (rIX-FP) has been developed, taking advantage of albumin fusion technology. As human albumin is an abundant plasma protein and does not act as a trigger for the immune system, it is an optimal partner to extend the half-life of coagulation factors. The unchanged recombinant FIX is fused to recombinant human albumin via a novel cleavable linker derived from the activation peptide of native FIX. The expressed fusion protein increases the circulating half-life of FIX and on activation of FIX during the physiologic blood coagulation albumin is removed through simultaneous cleavage of the linker. This study is proof of concept that rIX-FP effectively extended the circulating half-life of FIX in humans. Interestingly, the half-life in humans was even longer than the half-life that was previously measured in animal models.13
This first-in-human trial has demonstrated an excellent safety profile of rIX-FP. None of the subjects experienced thrombotic events or hypersensitivity reactions, the latter having previously been reported for both pdFIX and rFIX products.4,10,21 Furthermore, no inhibitors or non-neutralizing antibodies to FIX or albumin developed after rIX-FP exposure(s) in these previously treated subjects.
The hemostatic efficacy of coagulation factors is strictly related to their plasma concentration; thus, PK parameters are generally considered as important surrogate endpoints of efficacy of replacement therapy in hemophilia.18 Coagulation factor PK has important implications also with respect to prophylactic treatment because observational data supported the concept that the longer a patient spends with a low factor level the higher the risk of bleeding.22,23
In this light, the PK results of this phase 1 study with rIX-FP in 25 subjects with hemophilia B provide data that are of clinical interest. There was a 29% or 44% higher IR after infusion of 50 IU/kg rIX-FP compared with pdFIX or rFIX, respectively. The most outstanding features of rIX-FP are the prolonged circulation in plasma as shown by the long MRT and t1/2 (> 5-fold compared with the subjects' previous FIX products, at 50 IU/kg); the reduced CL; the small Vz, and the greater AUC (7-fold difference compared with previous FIX).
The relatively low IR seen with rFIX is dramatically improved, when comparing rIX-FP with rFIX, suggesting that the flow from plasma compartment to the extravascular space is more similar to the in vivo behavior of FIX. This is true not only just after the bolus administration but also for the entire decay curve, as indicated by a low Vss. The very low clearance, 16% and 14% of pdFIX or rFIX, respectively, should reduce the need for continuous or repeated bolus administration to maintain measurable trough levels > 5 IU/dL.
In this phase 1 trial with rIX-FP, the timing of PK assessment was prolonged up to 336 hours, as it was difficult to define a priori when FIX levels might return to baseline. The findings of higher than background, baseline-corrected trough levels of FIX 168 and 336 hours after rIX-FP administration (13.4 IU/dL and 7.4 IU/dL, respectively, for 50 IU/kg) are of great clinical interest. In addition, the corresponding high values of AUC0-inf are promising evidence for the potential to prolong the time interval between rIX-FP infusions and will be evaluated in future clinical trials.
To date, maintaining an adequate trough level is considered to be an important determinant of breakthrough bleeding,22,23 although other PK parameters (ie, AUC and peak levels) may also play a role under certain conditions. It is noteworthy that PK implications in the management of FIX prophylaxis are still theoretical because specific data are scanty in this clinical setting. In addition, the risk of bleeding may depend on other factors, such as lifestyle, physical activity, injuries, joint status, individual susceptibility, and response to replacement therapy; all these factors are already taken into account with individual dose tailoring and treatment optimization.
This study demonstrated the clinical safety and improved PK properties of a novel rFIX product, rIX-FP, which may permit prophylaxis with fewer intravenous injections per month. It may also permit a single injection to achieve and maintain hemostasis in the treatment of bleeding episodes. In addition, rIX-FP may also be able to provide and maintain high FIX trough levels, thus changing the patient's bleeding phenotype from severe to mild. Two larger clinical studies are currently underway to evaluate these anticipated improvements in the clinical care of hemophilia B.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank the investigators participating in the CSL654_2001 study for their contributions: Marc Trossaert, Center Régional de Traitement de l'Hémophilie, CHU Nantes, France; Uri Martinowitz, Israeli National Hemophilia Center, Chaim Sheba Medical Center, Tel Hashomer, Israel; Carmen Altisent, Hospital Universitari Vall d'Hebron Barcelona, Spain; Annarita Tagliaferri, Azienda Ospedaliera Universitaria di Parma, Parma, Italy; Chantal Rothschild, Hôpital Necker-CRTH, Paris, France; María Álvarez Román, H.U. La Paz, Madrid, Spain; Maria Fernanda López-Fernández, Complexo Hospitalario Universitario de A Coruna, A Coruna, Spain; Giancarlo Castaman, Ospedale San Bortolo, Vicenza, Italy; Thierry Lambert, CRTH Hôpital Bicêtre-Hémophiles, Kremlin-Bicêtre, France; Mario von Depka-Prondzinski, Werlhof Institute, Hannover, Germany; and Reinhard Schneppenheim, Universitätsklinikum Hamburg-Eppendorf, Hamburg, Germany. The authors also thank the study sub-investigators and coordinators who made this study possible; Dr Barbara Konkle, Pugent Sound Blood Center, Seattle, WA, chairperson of Data Review Committee; Rachael Easton, CSL Behring/United States for pharmacokinetic expert review and editorial support; Michelle Zanette and Deborah Rittenhouse, CSL Behring/United States for statistical analyses and programming management; Margaret Mitchell, Cindy Cochran, and Jay Newman, CSL Behring/United States for operation management; and Tina Moises and Stefanie Achenbach, CSL Behring/Germany for central laboratory testing support. The authors also thank the study subjects who participated without whose support this study would not have been possible.
This work was sponsored by CSL Behring, Marburg, Germany.
Authorship
Contribution: E.S. and M.M. designed and performed research, collected and interpreted data, and wrote and revised the manuscript; C.N. and R.K. designed and performed research, collected and interpreted data, and revised the manuscript; A.T. and I.P.F. performed research, collected and interpreted data, and revised the manuscript; and C.V. and I.J. designed the research, analyzed data, and revised the manuscript.
Conflict-of-interest disclosure: E.S., M.M., C.N., R.K., A.T., and I.P.F. received research support from CSL Behring to conduct the study, lecture fees, and honoraria for consultancy from CSL Behring. E.S. received fees as a speaker in meetings organized by Bayer, Baxter, Pfizer, NovoNordisk, Biotest, Kedrion, and Grifols, acted as a paid consultant for Pfizer, Baxter, Bayer, Kedrion, Grifols, and NovoNordisk, and received unrestricted research grants from NovoNordisk and Pfizer. C.V. and I.J. were employed at CSL Behring.
Correspondence: Elena Santagostino, Angelo Bianchi Bonomi Haemophilia and Thrombosis Centre, Istituto di Ricovero e Cura a Carattere Scientifico Cà Granda Foundation, Maggiore Hospital Policlinico, Via Pace 9, 20122 Milan, Italy; e-mail: elena.santagostino@policlinico.mi.it.