Abstract
Erythropoiesis is a robust process of cellular expansion and maturation occurring in murine bone marrow and spleen. We previously determined that sublethal irradiation, unlike bleeding or hemolysis, depletes almost all marrow and splenic erythroblasts but leaves peripheral erythrocytes intact. To better understand the erythroid stress response, we analyzed progenitor, precursor, and peripheral blood compartments of mice post–4 Gy total body irradiation. Erythroid recovery initiates with rapid expansion of late-stage erythroid progenitors–day 3 burst-forming units and colony-forming units, associated with markedly increased plasma erythropoietin (EPO). Although initial expansion of late-stage erythroid progenitors is dependent on EPO, this cellular compartment becomes sharply down-regulated despite elevated EPO levels. Loss of EPO-responsive progenitors is associated temporally with a wave of maturing erythroid precursors in marrow and with emergence of circulating erythroid progenitors and subsequent reestablishment of splenic erythropoiesis. These circulating progenitors selectively engraft and mature in irradiated spleen after short-term transplantation, supporting the concept that bone marrow erythroid progenitors migrate to spleen. We conclude that sublethal radiation is a unique model of endogenous stress erythropoiesis, with specific injury to the extravascular erythron, expansion and maturation of EPO-responsive late-stage progenitors exclusively in marrow, and subsequent reseeding of extramedullary sites.
Introduction
Erythropoiesis is a process of rapid cellular expansion and maturation that maintains the circulating red cell mass under steady-state conditions and in response to anemia. Anemia is a common side effect of radiation treatment, suggesting that the erythroid lineage is a highly sensitive target of ionizing radiation. It is known that circulating reticulocytes are severely depleted after sublethal total body irradiation (TBI) in mice.1,2 In addition, several studies have suggested that bone marrow progenitors and precursors are directly injured after radiation damage.3-7 Furthermore, we recently found that 4 Gy TBI rapidly induces the apoptosis of bone marrow erythroid progenitors and precursors, leading to severe depletion of bone marrow erythroblasts.8 Thus, radiation-induced erythroid stress, in which marrow erythroblasts are directly depleted and peripheral red cells relatively preserved, is nearly the opposite of more traditional erythroid stressors, such as bleeding or hemolysis in which the circulating red cell compartment is rapidly and severely lost but bone marrow erythroblasts are preserved.
Erythropoietin (EPO) is the central cytokine regulator of the erythroid lineage. The majority of steady-state erythropoiesis occurs in the bone marrow and is regulated by EPO-mediated survival and proliferation of late-stage erythroid progenitors and immature precursors.9-11 After pathologic damage that threatens oxygen tension, such as the acute loss of red blood cells (RBCs) by bleeding or hemolysis, EPO production is up-regulated, leading to the expansion of extramedullary erythropoiesis in the spleen of mice.12,13 This stress erythropoiesis is associated with high EPO levels, the expansion of stress erythroid progenitors in the spleen, and marked reduction in the rates of apoptosis normally seen in splenic erythroid progenitors.14-19 These processes provide a mechanism for the robust erythroid response necessary to ameliorate acute anemic stress.
During recovery from radiation injury, an increase in erythropoietic stimulatory activity leads to an expansion of EPO-responsive cells.6,20-22 In addition, EPO administered after radiation stimulates reticulocyte recovery and improves survival after lethal doses of radiation.23 These data suggest that, despite dramatically different target cells of depletion, recovery from sublethal radiation stress and from acute peripheral anemic stress may proceed through similar mechanisms of EPO-dependent erythroid cell expansion. Nevertheless, the specific cellular kinetics of recovery after sublethal radiation and the potential relationship with current concepts of stress erythropoiesis have not been specifically explored.
Here, we investigate the recovery of the erythroid lineage from acute sublethal radiation stress, using functional colony assays to study erythroid progenitors,24-27 including immature day 7 burst-forming units (d7 BFU-E) and more mature day 3 BFU-E (d3 BFU-E) and colony-forming units (CFU-E), and imaging flow cytometry to quantitate erythroblast precursors,8,28 including proerythroblasts (ProE) and basophilic (BasoE), polychromatophilic (PolyE), and orthochromatic (OrthoE) erythroblasts. We report that recovery of the erythron after sublethal radiation is centered on rapid expansion of bone marrow d3 BFU-E and CFU-E and that EPO is both necessary and sufficient for this initial in vivo response. Surprisingly, this expansion of EPO-responsive progenitors is followed by their rapid depletion despite elevated plasma EPO levels. The decline of late-stage erythroid progenitors is temporally associated with a robust wave of erythroid precursor maturation in the marrow and with transient egress of erythroid progenitors and precursors into the bloodstream. Unlike the rapid splenic expansion of erythropoiesis seen in response to peripheral anemic stress, the recovery of splenic erythropoiesis after acute radiation stress is delayed, occurring only after initial bone marrow recovery. Furthermore, we find that circulating erythroid progenitors selectively engraft the spleens of irradiated recipients after short-term transplantation, supporting the concept that erythroid progenitors migrate from marrow to spleen during recovery from radiation injury. Sublethal 4 Gy TBI serves as a useful model to investigate the endogenous recovery, migration, and extramedullary establishment of the erythroid lineage after clastogenic injury.
Methods
Animals and irradiation
Female 7- to 9-week-old C57BL/6J mice (The Jackson Laboratory) were irradiated while held in a Plexiglas restraint and exposed to 4 Gy TBI at a dose rate of 1.6 Gy/min from a Shepherd Irradiator with a 6000 Ci 137Cs source and collimating equipment. The University of Rochester Committee on Animal Resources approved all animal experiments and the study was conducted in accordance with the Declaration of Helsinki.
Blood, marrow, and spleen extraction
Mice were euthanized by CO2 narcosis and peripheral blood obtained. Bone marrow was harvested by flushing of femurs with PB2 (DPBS, Invitrogen; 0.3% BSA, Gemini Bio-Products; 0.68mM CaCl2, Sigma-Aldrich; 0.1% glucose) in 12.5 μg/mL heparin and single-cell suspensions made by trituration. Spleens were mechanically dissociated into single-cell suspensions in PB2. Marrow and spleen cell counts were obtained by hemacytometer.
Erythron analysis
Colony assays were performed to quantify erythroid progenitors as previously described.8 Briefly, single-cell suspensions of marrow or spleen were plated at 2 × 105 cells/mL for d7 BFU-E and d3 BFU-E and 1 × 105 cells/mL for CFU-E into methylcellulose media. CFU-E medium was supplemented with 0.3 U/mL rhEPO (Amgen), whereas d7 and d3 BFU-E media were supplemented with 2 U/mL rhEPO, 0.02 μg/mL IL-3 and IL-6, and 0.12 μg/mL SCF (Peprotech). CFU-E, d3 BFU-E, and d7 BFU-E were quantified as red cell colonies 2, 3, and 7 days after plating, respectively. Staining and analysis of erythroblast precursor subpopulations in marrow and spleen was performed using imaging flow cytometry (ImageStreamX, data collected using INSPIRE Version 4.1 and analyzed using IDEAS Version 4.0; Amnis) as recently described.8,28 Hematocrit and reticulocyte values on whole peripheral blood were obtained as previously described.8 For circulating erythroid progenitor and precursor quantification, whole peripheral blood was lysed in RBC lysis buffer (156mM NH4Cl, 127μM EDTA, 12mM NaHCO3), washed twice and resuspended in PB2, and analyzed.
Histology
Hindlimbs were removed and fixed in 4% formaldehyde for 24 hours, decalcified in 10% EDTA for 48 hours, and subsequently embedded in paraffin, sectioned at 5 μm, and H&E stained. Spleens were fixed in 4% formaldehyde for 24 hours and embedded, sectioned, and stained.
Plasma isolation and EPO assay
Plasma from C57BL/6 mice post–4 Gy TBI was collected by centrifugation of whole peripheral blood at 1000g for 20 minutes. EPO levels in plasma were determined by ELISA assay kit (R&D Systems).
RBC transfusions
C57BL/6 female age-matched donor mice were terminally bled and donor blood was washed and resuspended in PB2 at a 1:1 ratio. At 2 and 4 days post–4 Gy TBI, recipient mice were injected intraperitoneally with 500 μL of donor RBC/PB2 1:1 suspension or mock-transfused with 500 μL PB2. Peripheral blood was collected at 0, 2, 4, and 6 days after radiation and hematocrit and EPO levels determined. Marrow was isolated at 6 days after TBI and erythroid progenitors quantified.
Exogenous EPO injections
Post–4 Gy TBI, mice were injected intraperitoneally with 1000 IU/kg rhEPO in PB2 or mock-treated with PB2 alone at 1 hour, 4 days, or 10 days after radiation and analyzed at indicated time points. For EPO stimulation of unirradiated mice, exogenous EPO (1000 IU/kg) or PB2 was injected intraperitoneally, and marrow and spleen analyzed at 2 days after injection.
α4 and α5-integrin analysis
Post–4 Gy TBI, marrow and peripheral blood were harvested, and cells were blocked in 25% rat whole serum (Invitrogen) in PB2 and stained with PE-Cy7 ckit; APC-eFluor 780 B220, CD3, Gr1, CD11b, Ter119, Sca1, and CD16/32; PerCP eFluor 710 CD150; biotinylated CD105 (eBioscience); AF488 α5-integrin; and AF647 α4-integrin (BioLegend) at 1:100 dilution and DAPI at 5 μg/mL, secondarily stained with PE–Texas Red streptavidin at 1:500 dilution, and analyzed on the LSR II flow cytometer (BD Bioscience).
UBC-GFP short-term transplantation
UBC-GFP C57Bl/6 mice (The Jackson Laboratory) were exposed to 4 Gy TBI, and marrow and peripheral blood collected at 6 days after radiation. Nucleated cells were obtained from peripheral blood samples using Ficoll-Paque PLUS (GE Healthcare) separation. For stem and lineage-depletion, blocked UBC-GFP bone marrow cells were stained with biotinylated-B220, CD3, Gr1, CD11b, Ter119, Sca1, CD16/32 (eBioscience), and CD41 (AbD Serotec), incubated with IMag streptavidin magnetic particles (BD Bioscience), and magnetically separated using the iMagnet system (BD Bioscience). Approximately 2 × 105 depleted UBC-GFP hematopoietic progenitors from bone marrow or 2 × 104 depleted UBC-GFP hematopoietic progenitors from peripheral blood were intravenously injected into wild-type C57Bl/6 recipients at 6.5 days post–4 Gy TBI. At 12 hours after injection, marrow and spleen were isolated from recipient mice, dissociated into single-cell suspensions, stained with PE CD105, APC CD150, APC-eFluor 780 Ter119, PerCP-eFluor 710 ckit (eBioscience), and PE-Cy7 CD71 (BioLegend) at 1:100 dilution and DAPI at 5 μg/mL, and analyzed on the LSR II flow cytometer (BD Bioscience).
Results
Erythroid recovery after acute sublethal radiation stress is centered on late-stage erythroid progenitor expansion and maturation
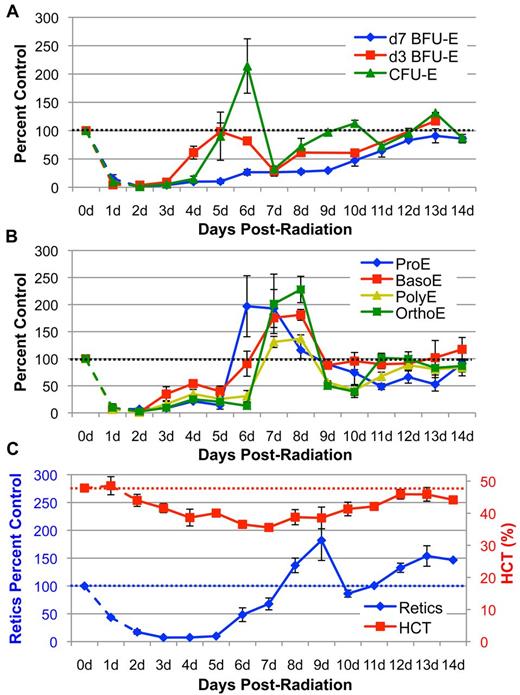
We previously determined that acute sublethal radiation stress (4 Gy TBI) leads to almost complete depletion of erythroid progenitors and precursors by 2 days after radiation (Figure 1A-B dotted lines).8 This depletion results in a rapid decrease in reticulocyte output and steadily decreasing peripheral RBC levels so that the hematocrit drops 9% by 4 days after radiation (Figure 1C). To systematically analyze the recovery of the erythroid progenitor and precursor compartments from 2 days to 14 days post–4 Gy TBI, we used colony assays (supplemental Figure 1, available on the Blood Web site; see the Supplemental Materials link at the top of the online article)8,29 and imaging flow cytometry,8,28 respectively. As shown in Figure 1A, levels of the most immature erythroid-specific progenitors, d7 BFU-E, recover very slowly and do not reach normal levels even by 14 days after TBI. In sharp contrast, d3 BFU-E numbers expand nearly 7-fold between 3 and 4 days after radiation and continue to increase at 5 days after TBI (Figure 1A). This expansion of d3 BFU-E directly precedes an even greater increase in CFU-E numbers, which expand more than 10-fold at days 4 to 6 after radiation and reach greater than 200% of unirradiated control levels (Figure 1A). These results indicate that late-stage erythroid progenitors, consisting of d3 BFU-E and CFU-E, are preferentially stimulated to expand during the initial recovery from sublethal radiation injury.
Kinetics of erythroid recovery in the 3 compartments of the erythron after sublethal 4 Gy TBI. (A) Erythroid progenitor (d7 BFU-E, d3 BFU-E, CFU-E) kinetics in the bone marrow post–4 Gy TBI. Recovery initiates in the d3 BFU-E progenitor at 4 days after radiation followed by rapid expansion of CFU-E at 5 to 6 days after TBI. (B) Erythroblast precursor (ProE, BasoE, PolyE, OrthoE) kinetics in the bone marrow post–4 Gy TBI. Erythroid precursor recovery follows expansion of late-stage erythroid progenitors. Progenitors and precursors are normalized per femur and expressed as a percent of unirradiated control marrow. (C) Hematocrit (HCT) and reticulocyte (Retic) levels in the peripheral blood post–4 Gy TBI. Circulating red cell recovery occurs by 8 to 10 days in reticulocytes and by 14 days in RBC. Reticulocytes are calculated as absolute reticulocyte index (percent Retic × total RBC and Retic number × %HCT) and expressed as a percent of unirradiated control levels. RBC levels are expressed as percent HCT. Dotted lines represent unirradiated control levels. Dashed data points at 1 to 2 days after radiation represent erythroid injury data previously published8 and shown here for clarity. Error bars represent SEM of at least 3 experiments, and 3 or more independently assayed mice were used to determine each data point.
Kinetics of erythroid recovery in the 3 compartments of the erythron after sublethal 4 Gy TBI. (A) Erythroid progenitor (d7 BFU-E, d3 BFU-E, CFU-E) kinetics in the bone marrow post–4 Gy TBI. Recovery initiates in the d3 BFU-E progenitor at 4 days after radiation followed by rapid expansion of CFU-E at 5 to 6 days after TBI. (B) Erythroblast precursor (ProE, BasoE, PolyE, OrthoE) kinetics in the bone marrow post–4 Gy TBI. Erythroid precursor recovery follows expansion of late-stage erythroid progenitors. Progenitors and precursors are normalized per femur and expressed as a percent of unirradiated control marrow. (C) Hematocrit (HCT) and reticulocyte (Retic) levels in the peripheral blood post–4 Gy TBI. Circulating red cell recovery occurs by 8 to 10 days in reticulocytes and by 14 days in RBC. Reticulocytes are calculated as absolute reticulocyte index (percent Retic × total RBC and Retic number × %HCT) and expressed as a percent of unirradiated control levels. RBC levels are expressed as percent HCT. Dotted lines represent unirradiated control levels. Dashed data points at 1 to 2 days after radiation represent erythroid injury data previously published8 and shown here for clarity. Error bars represent SEM of at least 3 experiments, and 3 or more independently assayed mice were used to determine each data point.
After the rapid expansion of late-stage erythroid progenitors at 4 to 6 days after radiation, d3 BFU-E and CFU-E are sharply down-regulated to 30% of control levels between 6 to 7 days after radiation (Figure 1A). This unexpected, rapid drop in late-stage erythroid progenitor numbers, however, is temporally associated with a robust wave of maturing erythroid precursors, starting with ProE and BasoE at 6 to 7 days after radiation that mature into PolyE and OrthoE at 7 to 8 days after radiation (Figure 1B). This wave of maturing erythroblasts is followed by a peak in reticulocyte output in the bloodstream at 8 to 9 days after radiation and a subsequent increase in hematocrit at 10 to 13 days after radiation (Figure 1C). Histologic analysis of marrow correlated with our quantitation of erythroid progenitors and precursors. At 2 days after radiation, there is massive depletion of nucleated cells accompanied by vascular dilation (supplemental Figure 2). With the recovery of erythroid precursors at 7 days after radiation, near-maximal filling of the marrow space is evident. By 13 days after TBI, marrow architecture and cellularity have normalized to steady-state levels (supplemental Figure 2). Taken together, these data indicate that expansion of late-stage erythroid progenitors initiates a subsequent wave of terminal maturation that leads to erythroid recovery in marrow and peripheral blood after acute radiation stress.
Increased endogenous plasma EPO levels are temporally correlated with expansion of d3 BFU-E and CFU-E levels
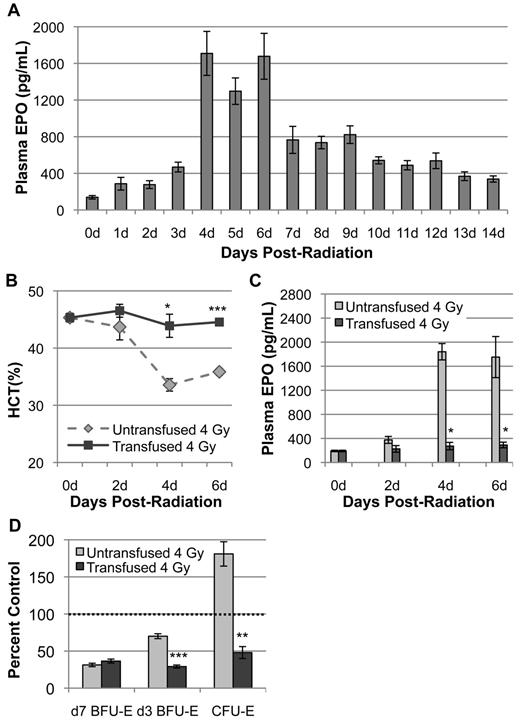
During steady-state erythropoiesis and in times of acute stress, EPO acts on CFU-E and ProE to promote their survival and proliferation.9-11,30 However, recent studies have shown that EPO-receptor expression and responsiveness initiates in a pre-CFU-E hematopoietic population, suggesting that d3 BFU-E may also be EPO-responsive in vivo.31,32 Therefore, we asked whether EPO regulates the specific increase in d3 BFU-E and CFU-E numbers after acute radiation stress. Analysis of plasma EPO levels post–4 Gy TBI reveals that endogenous EPO gradually increases 2- to 3-fold by 3 days after radiation. However, at 4 days after TBI, EPO levels increase to 13-fold more than control values (Figure 2A). These significantly elevated values are maintained through 6 days after radiation. A moderate drop in EPO levels occurs at 7 days, followed by a gradual decrease to 14 days after TBI (Figure 2A). This robust spike in endogenous EPO at 4 days post–4 Gy TBI correlates temporally with the rapid increase of d3 BFU-E at 4 to 5 days and CFU-E at 5 to 6 days after radiation (Figure 1A). These data suggest that EPO may act on d3 BFU-E and CFU-E to initiate their expansion and drive subsequent repopulation of bone marrow after radiation injury.
Endogenous EPO induction by anemia is required for expansion of d3 BFU-E and CFU-E during recovery from 4 Gy TBI. (A) Endogenous plasma EPO levels, expressed in pg/mL, as determined by ELISA at 0 to 14 days post–4 Gy TBI. EPO levels increase 13-fold at 4 days post–4 Gy TBI and remain at high levels through 6 days after radiation. (B) Hematocrit levels post–4 Gy TBI ± transfusion of washed RBC at 2 days and 4 days after radiation. Transfusion of irradiated mice prevents radiation-induced anemia. (C) Plasma EPO levels post–4 Gy TBI ± transfusion. Transfusion blocks the induction of EPO normally seen at 4 days and 6 days after radiation. For all experiments, plasma EPO levels and HCT were performed in triplicate with 3 independent blood samples. For each EPO and HCT determination, plasma was isolated from a single terminally bled mouse. All mice were euthanized mid-morning. (D) Erythroid bone marrow progenitor expansion at 6 days after radiation ± transfusion, normalized per femur, and expressed as a percent of unirradiated control marrow. The transfusion-induced block of EPO induction abrogates d3 BFU-E and CFU-E expansion but has no effect on d7 BFU-E during erythroid recovery from 4 Gy TBI. Dotted lines represent unirradiated control levels. Error bars represent SEM of at least 3 experiments, and 3 or more independently assayed mice were used to determine each data point. Statistical analyses were performed using a 2-tailed Student t test (*P < .05; **P < .01; ***P < .001; significantly different from 4 Gy TBI untransfused mice at matched time points).
Endogenous EPO induction by anemia is required for expansion of d3 BFU-E and CFU-E during recovery from 4 Gy TBI. (A) Endogenous plasma EPO levels, expressed in pg/mL, as determined by ELISA at 0 to 14 days post–4 Gy TBI. EPO levels increase 13-fold at 4 days post–4 Gy TBI and remain at high levels through 6 days after radiation. (B) Hematocrit levels post–4 Gy TBI ± transfusion of washed RBC at 2 days and 4 days after radiation. Transfusion of irradiated mice prevents radiation-induced anemia. (C) Plasma EPO levels post–4 Gy TBI ± transfusion. Transfusion blocks the induction of EPO normally seen at 4 days and 6 days after radiation. For all experiments, plasma EPO levels and HCT were performed in triplicate with 3 independent blood samples. For each EPO and HCT determination, plasma was isolated from a single terminally bled mouse. All mice were euthanized mid-morning. (D) Erythroid bone marrow progenitor expansion at 6 days after radiation ± transfusion, normalized per femur, and expressed as a percent of unirradiated control marrow. The transfusion-induced block of EPO induction abrogates d3 BFU-E and CFU-E expansion but has no effect on d7 BFU-E during erythroid recovery from 4 Gy TBI. Dotted lines represent unirradiated control levels. Error bars represent SEM of at least 3 experiments, and 3 or more independently assayed mice were used to determine each data point. Statistical analyses were performed using a 2-tailed Student t test (*P < .05; **P < .01; ***P < .001; significantly different from 4 Gy TBI untransfused mice at matched time points).
Prevention of radiation-induced anemia blocks EPO release and abrogates subsequent d3 BFU-E and CFU-E expansion
We next asked whether EPO was required for late-stage erythroid progenitor expansion after radiation injury. Because EPO levels increase in response to decreased peripheral oxygen tension,30,33 we asked whether the EPO induction post–4 Gy TBI occurs in response to the gradual development of anemia that occurs over the first 4 days after radiation (Figure 1C). EPO loss-of-function studies were performed by RBC transfusion at 2 days and 4 days post–4 Gy TBI. Transfused irradiated mice maintained a normal hematocrit (44%-47%) during the first 6 days post–4 Gy TBI (Figure 2B). These transfused mice also failed to increase EPO levels at 4 and 6 days after radiation (Figure 2C), indicating that the mild radiation-induced anemia is responsible for the increased production of endogenous EPO after sublethal TBI. Furthermore, analysis of erythroid progenitor kinetics at the apex of endogenous erythroid progenitor expansion (6 days after radiation) reveals that d3 BFU-E and CFU-E expansion is significantly abrogated in transfused mice, with minimal recovery of these populations to less than 50% of unirradiated control (Figure 2D). In contrast, the slow, linear recovery of d7 BFU-E during this time period is not affected by transfusion (Figure 2D), indicating that d7 BFU-E are not responsive to EPO stimulation. Overall, these findings indicate that, after acute radiation stress, EPO is required for the specific expansion of late-stage erythroid progenitors that leads to recovery of the erythron.
EPO supplementation post–4 Gy TBI significantly accelerates erythroid recovery and mirrors the endogenous response
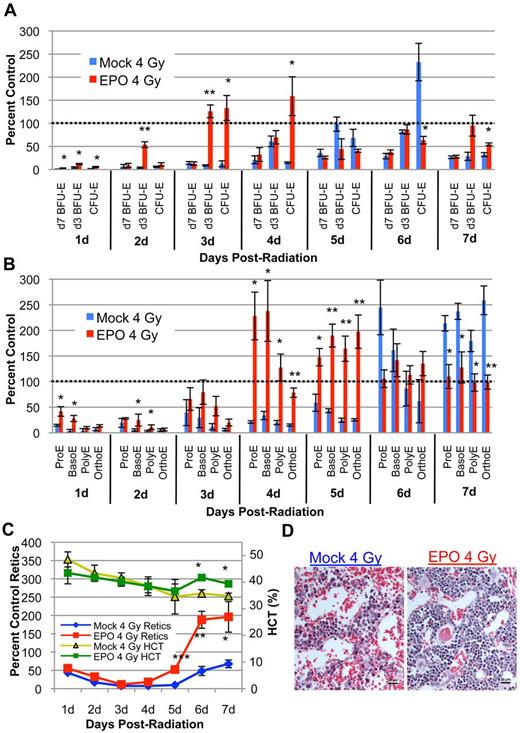
As the highest levels of plasma EPO are not present until 4 days post–4 Gy TBI, we performed gain-of-function studies to determine whether exogenous EPO administered at 1 hour after radiation could directly stimulate the expansion of d3 BFU-E and CFU-E. Erythroid recovery in irradiated, EPO-treated mice initiates in the marrow as d3 BFU-E and CFU-E expand as early as 2 and 3 days after TBI, respectively (Figure 3A red bars). Thus, exogenous EPO specifically accelerates late-stage erythroid progenitor recovery by 2 to 3 days compared with endogenous progenitor recovery in irradiated, mock-treated mice (Figure 3A blue bars). The accelerated wave of late-stage erythroid progenitors in irradiated, EPO-treated mice gives rise to a wave of maturing erythroid precursors at 4 to 5 days after radiation (Figure 3B red bars) and to reticulocytes and RBC in the peripheral blood by 5 to 6 days after radiation (Figure 3C red and green lines). Histologic examination of the marrow after radiation reveals marked erythroid reconstitution and decreased vascular dilation in EPO-treated mice compared with 4 Gy TBI mock-treated mice, indicative of accelerated erythroid marrow recovery (Figure 3D). These data indicate that a single dose of exogenous EPO after acute radiation stress not only accelerates expansion of late-stage erythroid progenitors but also drives robust maturation and recovery of all downstream erythroid subpopulations. In addition, the accelerated synchronous wave of recovery after exogenous EPO treatment (Figure 3A-C red bars) closely mirrors the physiologic wave of recovery during the endogenous erythroid response (Figure 1A-C; Figure 3A-C blue bars), suggesting that EPO is not only necessary, but also sufficient to drive the expansion of d3 BFU-E and CFU-E and initiate recovery of the erythron after sublethal TBI.
Exogenous EPO is sufficient to initiate late-stage erythroid progenitor expansion and accelerate erythroid recovery after sublethal irradiation. (A) Erythroid progenitor recovery kinetics in the bone marrow ± intraperitoneal injection of 1000 IU/kg EPO at 1 hour post–4 Gy TBI. Recovery of d3 BFU-E is advanced by 2 to 3 days in EPO-injected mice (red bars) compared with mock-treated mice (blue bars) and leads to accelerated CFU-E recovery. (B) Erythroid bone marrow precursor recovery kinetics ± IP EPO injection at 1 hour after TBI. Erythroid precursors in EPO-injected mice (red bars) undergo a wave of recovery 2 to 3 days sooner than mock-treated mice (blue bars) with kinetics that mirror endogenous recovery. Erythroid progenitors and precursors are normalized per femur and expressed as a percent of unirradiated control marrow. (C) Circulating red cell recovery ± EPO injection at 1 hour post–4 Gy TBI. Advanced reticulocyte recovery beginning at 5 days after radiation (red line) leads to partial HCT normalization by 6 days after TBI (green line) in EPO-treated mice. Reticulocytes are calculated as absolute reticulocyte index (% Retic × total RBC and Retic number × %HCT) and expressed as a percent of unirradiated control levels. RBC levels are expressed as percent HCT. Dotted lines represent unirradiated control levels. Error bars represent SEM of at least 3 experiments, and 3 or more independently assayed mice were used to determine each data point. Statistical analyses were performed using a 2-tailed Student t test, (*P < .05; **P < .01; ***P < .001; significantly different from 4 Gy TBI mock-treated mice at matched timepoints). (D) Representative histologic sections of bone marrow ± EPO 4 days post–4 Gy TBI (H&E; 20-micron bars). EPO treatment leads to increased cellularity and decreased vascular dilation compared with mock-treated samples. Images were captured with a Nikon Digital Sight Ds-Fi1 camera using Nikon NIS-Elements software on a Nikon Eclipse 80i upright microscope using a 20× objective.
Exogenous EPO is sufficient to initiate late-stage erythroid progenitor expansion and accelerate erythroid recovery after sublethal irradiation. (A) Erythroid progenitor recovery kinetics in the bone marrow ± intraperitoneal injection of 1000 IU/kg EPO at 1 hour post–4 Gy TBI. Recovery of d3 BFU-E is advanced by 2 to 3 days in EPO-injected mice (red bars) compared with mock-treated mice (blue bars) and leads to accelerated CFU-E recovery. (B) Erythroid bone marrow precursor recovery kinetics ± IP EPO injection at 1 hour after TBI. Erythroid precursors in EPO-injected mice (red bars) undergo a wave of recovery 2 to 3 days sooner than mock-treated mice (blue bars) with kinetics that mirror endogenous recovery. Erythroid progenitors and precursors are normalized per femur and expressed as a percent of unirradiated control marrow. (C) Circulating red cell recovery ± EPO injection at 1 hour post–4 Gy TBI. Advanced reticulocyte recovery beginning at 5 days after radiation (red line) leads to partial HCT normalization by 6 days after TBI (green line) in EPO-treated mice. Reticulocytes are calculated as absolute reticulocyte index (% Retic × total RBC and Retic number × %HCT) and expressed as a percent of unirradiated control levels. RBC levels are expressed as percent HCT. Dotted lines represent unirradiated control levels. Error bars represent SEM of at least 3 experiments, and 3 or more independently assayed mice were used to determine each data point. Statistical analyses were performed using a 2-tailed Student t test, (*P < .05; **P < .01; ***P < .001; significantly different from 4 Gy TBI mock-treated mice at matched timepoints). (D) Representative histologic sections of bone marrow ± EPO 4 days post–4 Gy TBI (H&E; 20-micron bars). EPO treatment leads to increased cellularity and decreased vascular dilation compared with mock-treated samples. Images were captured with a Nikon Digital Sight Ds-Fi1 camera using Nikon NIS-Elements software on a Nikon Eclipse 80i upright microscope using a 20× objective.
Splenic erythropoiesis is delayed until after initial bone marrow recovery
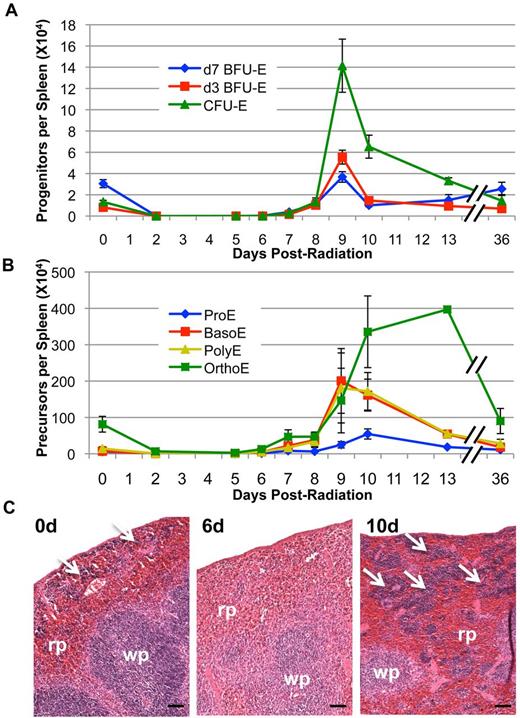
As spleen is known to play an important role in the murine erythroid stress response after bleeding or hemolysis,12,13 we asked whether extramedullary erythropoiesis also occurs in the spleen after acute radiation stress. Surprisingly, we find that splenic erythropoiesis is absent during initial recovery of erythroid progenitors and precursors in the bone marrow at 4 to 6 days after TBI (Figure 4A-B). However, a massive expansion of erythroid progenitors, particularly CFU-E, begins in the spleen at 7 days after radiation and peaks at 9 days after TBI (Figure 4A). This erythroid progenitor recovery is accompanied by erythroblast precursor expansion that also initiates at 7 to 8 days after radiation and continues to at least 13 days post–4 Gy TBI (Figure 4B). Furthermore, histologic analysis reveals that the basal level of splenic erythropoiesis (Figure 4C white arrows) is absent even at 6 days after radiation, despite rapid marrow recovery occurring at this same time point (Figure 4C; Figure 1A-B). However, massive expansion of the red pulp occurs by 10 days after radiation, indicating a rapid induction of extramedullary erythropoiesis in the spleen (Figure 4C white arrows). These data indicate that, unlike the acute anemic stress response to bleeding or hemolysis in which erythroid progenitors in the spleen initiate recovery,17,34 splenic erythropoiesis after acute radiation stress is delayed until after recovery of erythropoiesis in the bone marrow.
Splenic erythroid progenitor and precursor recovery post–4 Gy TBI. (A) Erythroid progenitor kinetics in the spleen post–4 Gy TBI. Splenic erythroid progenitor recovery does not begin until 7 to 8 days after radiation and peaks at 9 days after TBI. (B) Splenic erythroid precursor kinetics post–4 Gy TBI. Erythroid precursor recovery begins by 8 to 9 days in spleen and remains at high levels, especially in later precursors, at 13 days after TBI. Erythroid progenitors and precursors are expressed as the total number of each cell type per spleen. Error bars represent SEM of at least 3 experiments, and 3 or more independently assayed mice were used to determine each data point. (C) Representative sections of unirradiated spleen and spleen at 6 and 10 days post–4 Gy TBI (H&E staining; 50-micron bars). The low level of steady-state erythropoiesis in spleen is rapidly lost and remains absent at 6 days after radiation; robust erythropoiesis occurs exclusively in the red pulp by 10 days post–4 Gy TBI. White arrows represent areas of erythroid activity in the spleen; rp indicates red pulp; and wp, white pulp. Images were captured with a Nikon Digital Sight Ds-Fi1 camera using Nikon NIS-Elements software on a Nikon Eclipse 80i upright microscope using a 10× objective.
Splenic erythroid progenitor and precursor recovery post–4 Gy TBI. (A) Erythroid progenitor kinetics in the spleen post–4 Gy TBI. Splenic erythroid progenitor recovery does not begin until 7 to 8 days after radiation and peaks at 9 days after TBI. (B) Splenic erythroid precursor kinetics post–4 Gy TBI. Erythroid precursor recovery begins by 8 to 9 days in spleen and remains at high levels, especially in later precursors, at 13 days after TBI. Erythroid progenitors and precursors are expressed as the total number of each cell type per spleen. Error bars represent SEM of at least 3 experiments, and 3 or more independently assayed mice were used to determine each data point. (C) Representative sections of unirradiated spleen and spleen at 6 and 10 days post–4 Gy TBI (H&E staining; 50-micron bars). The low level of steady-state erythropoiesis in spleen is rapidly lost and remains absent at 6 days after radiation; robust erythropoiesis occurs exclusively in the red pulp by 10 days post–4 Gy TBI. White arrows represent areas of erythroid activity in the spleen; rp indicates red pulp; and wp, white pulp. Images were captured with a Nikon Digital Sight Ds-Fi1 camera using Nikon NIS-Elements software on a Nikon Eclipse 80i upright microscope using a 10× objective.
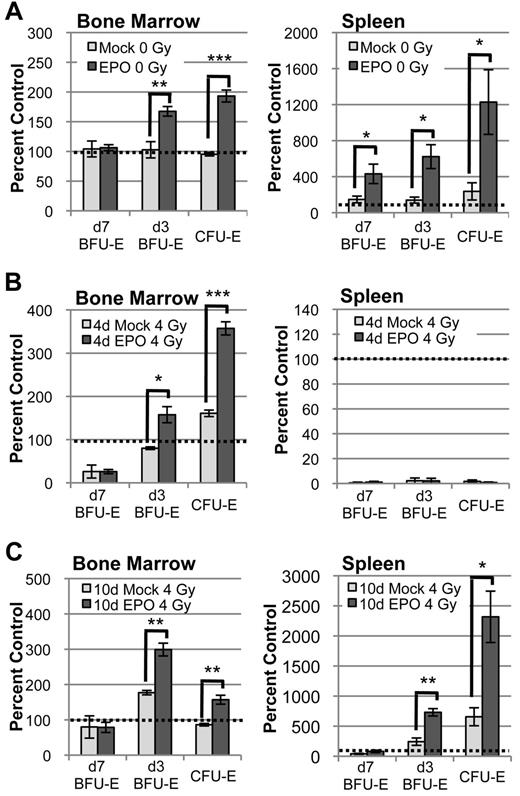
Under normal steady-state conditions, EPO supplementation stimulates robust late-stage erythroid progenitor expansion both in the bone marrow and in the spleen (Figure 5A). To test functionally whether there are any EPO-responsive progenitors present in the bone marrow and spleen during the initial recovery from acute radiation stress, we administered exogenous EPO at 4 days after radiation, supplementing EPO levels at the peak of the endogenous EPO response. This EPO treatment specifically stimulates bone marrow d3 BFU-E to further expand to more than 150% and CFU-E to expand to more than 300% of unirradiated control by 6 days after radiation, with no effect on the d7 BFU-E population (Figure 5B left panel). In contrast, exogenous EPO administered at 4 days after TBI does not alter the profound lack of erythroid progenitors in the spleen (Figure 5B right panel). However, EPO injection at 10 days after radiation drives the expansion of d3 BFU-E and CFU-E both in the marrow and in the spleen (Figure 5C). Taken together, these data indicate that, after acute radiation stress, functional late-stage erythroid progenitors are not present in the spleen at the time of rapid bone marrow expansion and that extramedullary erythropoiesis reinitiates only after recovery of the erythron in the marrow.
EPO-responsive progenitors are not present in the spleen until after their recovery in the bone marrow. (A) Erythroid progenitor kinetics in bone marrow and spleen 2 days after IP injection of 1000 IU/kg EPO in unirradiated mice. At steady-state, EPO-responsive progenitors are present in both bone marrow and spleen and rapidly expand in response to EPO stimulation. (B) Erythroid progenitor kinetics in bone marrow and spleen at 6 days after radiation ± IP EPO injection at 4 days post–4 Gy TBI. Late-stage erythroid progenitors expand in bone marrow but do not expand in spleen, indicating that EPO-responsive progenitors are not present in spleen during the period of rapid marrow recovery. (C) Erythroid progenitor kinetics in bone marrow and spleen at 12 days after radiation ± IP EPO injection at 10 days post–4 Gy TBI. EPO responsive progenitors are present in spleen by 10 days after radiation, consistent with delayed initiation of splenic expansion. Progenitors are normalized per femur or spleen and expressed as a percent of unirradiated control. Dotted lines represent unirradiated control levels in all graphs. Error bars represent SEM of at least 3 experiments, and 3 or more independently assayed mice were used to determine each data point. Statistical analyses were performed using a 2-tailed Student t test (*P < .05; **P < .01; ***P < .001; significantly different from mock-treated mice at matched time points).
EPO-responsive progenitors are not present in the spleen until after their recovery in the bone marrow. (A) Erythroid progenitor kinetics in bone marrow and spleen 2 days after IP injection of 1000 IU/kg EPO in unirradiated mice. At steady-state, EPO-responsive progenitors are present in both bone marrow and spleen and rapidly expand in response to EPO stimulation. (B) Erythroid progenitor kinetics in bone marrow and spleen at 6 days after radiation ± IP EPO injection at 4 days post–4 Gy TBI. Late-stage erythroid progenitors expand in bone marrow but do not expand in spleen, indicating that EPO-responsive progenitors are not present in spleen during the period of rapid marrow recovery. (C) Erythroid progenitor kinetics in bone marrow and spleen at 12 days after radiation ± IP EPO injection at 10 days post–4 Gy TBI. EPO responsive progenitors are present in spleen by 10 days after radiation, consistent with delayed initiation of splenic expansion. Progenitors are normalized per femur or spleen and expressed as a percent of unirradiated control. Dotted lines represent unirradiated control levels in all graphs. Error bars represent SEM of at least 3 experiments, and 3 or more independently assayed mice were used to determine each data point. Statistical analyses were performed using a 2-tailed Student t test (*P < .05; **P < .01; ***P < .001; significantly different from mock-treated mice at matched time points).
Erythroid progenitors and precursors transiently emerge into the bloodstream after bone marrow recovery and before re-initiation of splenic erythropoiesis
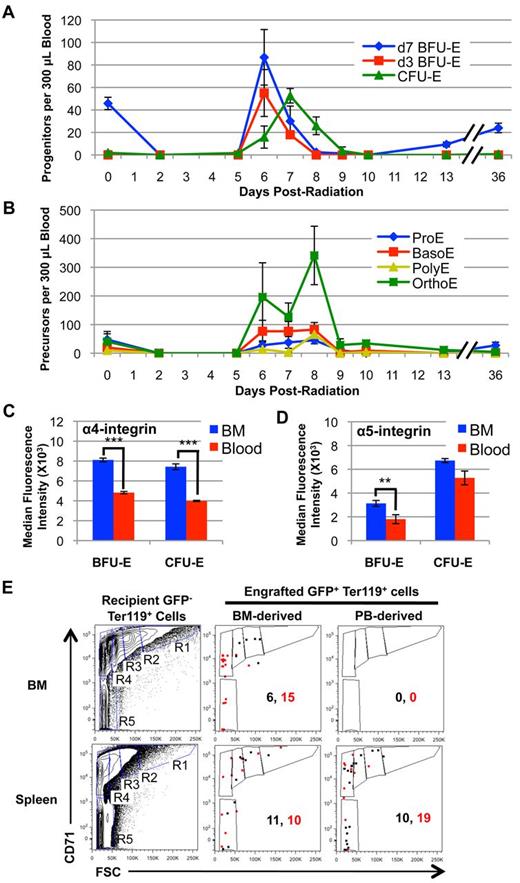
The lack of functional erythroid progenitors in the spleen during initial bone marrow recovery (Figure 5B) suggests that the cellular source for re-initiation of splenic erythropoiesis originates outside of the spleen. We have found that d3 BFU-E and CFU-E levels are sharply down-regulated between 6 and 7 days after radiation, immediately after late-stage progenitor expansion (Figure 1A). Although much of this down-regulation is probably because of the maturation of these progenitors into downstream precursors and ultimately RBCs (Figures 1B-C), we asked whether erythroid progenitors also circulate in the bloodstream and migrate to spleen during this critical time point after marrow recovery and before splenic expansion. At steady state, d7 BFU-E are the most frequent circulating nucleated erythroid cells, with only rare d3 BFU-E, CFU-E and erythroblast precursors present in the bloodstream (Figure 6A-B). Post–4 Gy TBI, all erythroid progenitors and precursors are rapidly depleted from the bloodstream and are not evident at 5 days after radiation. However, a large number of erythroid progenitors and precursors emerge into the bloodstream at 6 days after TBI and persist until 8 to 9 days after radiation (Figure 6A-B). Given their relative low levels in the marrow at 6 days after TBI (Figure 1A), d7 BFU-E are disproportionally localized in the bloodstream (Figure 6A). These data support the concept that d7 BFU-E are the most mobile cells of the extravascular erythron. Importantly, this transient wave of circulating erythroid progenitors occurs after erythropoiesis has recovered in the marrow but before re-initiation of erythropoiesis in the spleen (Figures 1A-B, 4A-B, and 6A-B), consistent with a model of endogenous migration of the erythron from bone marrow to spleen during recovery from acute radiation stress.
A transient wave of erythroid progenitors circulates in the bloodstream at 6 to 8 days post–4 Gy TBI and selectively engrafts spleen. (A) Erythroid progenitor kinetics in the peripheral blood post–4 Gy TBI. Erythroid progenitors (predominantly d7 BFU-E) are completely lost from the bloodstream after irradiation. A transient wave of erythroid progenitors emerges into the bloodstream between 6 and 8 days after radiation. (B) Erythroid precursor kinetics in the peripheral blood post–4 Gy TBI. Erythroid precursors are also lost after irradiation and transiently emerge into the bloodstream at 6 to 9 days after radiation. Erythroid progenitors and precursors are expressed as the total number of each subpopulation per 300 μL whole blood. (C) Median fluorescence intensity of surface α4-integrin levels on BFU-E and CFU-E obtained from the bone marrow (BM; blue) and circulating blood (red) of mice at 6 days post–4 Gy TBI. α4-integrin levels are significantly decreased on both BFU-E and CFU-E in the bloodstream compared with bone marrow erythroid progenitors at 6 days after radiation. (D) Median fluorescence intensity of surface α5-integrin levels on BFU-E and CFU-E obtained from the bone marrow (BM; blue) and circulating blood (red) of mice at 6 days post–4 Gy TBI. Error bars represent SEM of 3 or more independently assayed mice for each data point. Statistical analyses were performed using a 2-tailed Student t test (**P < .01; ***P < .001; significantly different from 6 day post-TBI BM progenitors at matched time points). (E) Flow cytometric analysis of erythroid progenitor engraftment in irradiated recipient BM and spleen at 12 hours after intravenous injection of Sca1−CD16/32− lineage-depleted donor UBC-GFP hematopoietic progenitors from BM and peripheral blood (PB) isolated at 6 days post–4 Gy TBI. Progenitors from bone marrow of UBC-GFP mice at 6 days post–4 Gy TBI can successfully engraft and mature into the bone marrow and spleen of 6.5 day post-TBI recipient mice (center column). In contrast, progenitors isolated from the peripheral blood of UBC-GFP at 6 days post–4 Gy TBI selectively engraft the spleen of recipient mice (right column). GFP− Ter119+ recipient BM and spleen cells were used to gate subpopulations of maturing erythroblasts using CD71 and forward scatter characteristics18 (left column), with R1 representing the most immature and R5 the most mature erythroid subpopulations. Data are shown from 2 independent experiments (shown in black and red), with numbers of GFP+ Ter119+ donor cells present in recipient marrow and spleen per 2 × 106 cells analyzed.
A transient wave of erythroid progenitors circulates in the bloodstream at 6 to 8 days post–4 Gy TBI and selectively engrafts spleen. (A) Erythroid progenitor kinetics in the peripheral blood post–4 Gy TBI. Erythroid progenitors (predominantly d7 BFU-E) are completely lost from the bloodstream after irradiation. A transient wave of erythroid progenitors emerges into the bloodstream between 6 and 8 days after radiation. (B) Erythroid precursor kinetics in the peripheral blood post–4 Gy TBI. Erythroid precursors are also lost after irradiation and transiently emerge into the bloodstream at 6 to 9 days after radiation. Erythroid progenitors and precursors are expressed as the total number of each subpopulation per 300 μL whole blood. (C) Median fluorescence intensity of surface α4-integrin levels on BFU-E and CFU-E obtained from the bone marrow (BM; blue) and circulating blood (red) of mice at 6 days post–4 Gy TBI. α4-integrin levels are significantly decreased on both BFU-E and CFU-E in the bloodstream compared with bone marrow erythroid progenitors at 6 days after radiation. (D) Median fluorescence intensity of surface α5-integrin levels on BFU-E and CFU-E obtained from the bone marrow (BM; blue) and circulating blood (red) of mice at 6 days post–4 Gy TBI. Error bars represent SEM of 3 or more independently assayed mice for each data point. Statistical analyses were performed using a 2-tailed Student t test (**P < .01; ***P < .001; significantly different from 6 day post-TBI BM progenitors at matched time points). (E) Flow cytometric analysis of erythroid progenitor engraftment in irradiated recipient BM and spleen at 12 hours after intravenous injection of Sca1−CD16/32− lineage-depleted donor UBC-GFP hematopoietic progenitors from BM and peripheral blood (PB) isolated at 6 days post–4 Gy TBI. Progenitors from bone marrow of UBC-GFP mice at 6 days post–4 Gy TBI can successfully engraft and mature into the bone marrow and spleen of 6.5 day post-TBI recipient mice (center column). In contrast, progenitors isolated from the peripheral blood of UBC-GFP at 6 days post–4 Gy TBI selectively engraft the spleen of recipient mice (right column). GFP− Ter119+ recipient BM and spleen cells were used to gate subpopulations of maturing erythroblasts using CD71 and forward scatter characteristics18 (left column), with R1 representing the most immature and R5 the most mature erythroid subpopulations. Data are shown from 2 independent experiments (shown in black and red), with numbers of GFP+ Ter119+ donor cells present in recipient marrow and spleen per 2 × 106 cells analyzed.
Circulating BFU-E and CFU-E express lower levels of α4 and α5-integrins compared with bone marrow erythroid progenitors at 6 days after radiation
α4 and α5-integrins are known to mediate macrophage-erythroid interactions within the context of the microenvironmental erythroblast island.35,36 In addition, recent studies have demonstrated an important role for α4β1 and α5β1-integrins in stress erythropoiesis.37 We therefore asked whether integrin expression differed on the surface of erythroid progenitors located in the bone marrow versus the bloodstream at 6 days after radiation. We specifically analyzed α4 and α5-integrin levels on BFU-E and CFU-E using a flow cytometric approach (supplemental Figure 3A).38,39 As shown in Figure 6C and supplemental Figure 3B, α4-integrin levels on circulating BFU-E and CFU-E are significantly lower than those on erythroid progenitors in the marrow at 6 days after TBI. In addition, α5-integrin levels are moderately decreased on circulating BFU-E compared with marrow progenitors (Figure 6D, supplemental Figure 3C). These data suggest that down-regulation of integrins on erythroid progenitors may play a role in their egress from the marrow into the bloodstream.
Circulating erythroid progenitors at 6 days after radiation selectively engraft and mature in spleen
Our kinetic studies (Figure 6A) suggest that circulating erythroid progenitors at 6 days after TBI are responsible for the re-initiation of extramedullary erythropoiesis in the spleen. To test this hypothesis, we used a short-term transplant assay with UBC-GFP mice40 serving as donors and C57Bl/6 mice as recipients. Sca1− CD16/32− lineage-depleted progenitors isolated from the bone marrow and from the peripheral blood of UBC-GFP donor mice at 6 days after TBI were injected into C57Bl/6 mice at 6.5 days after radiation, and marrow and splenic engraftment was assayed 12 hours later by flow cytometry. GFP+ Ter119+ cells were identified in recipient marrow and spleen (supplemental Figure 4A-B) and gated on forward scatter and CD7118 to further determine the identity of the engrafted cells (Figure 6E). We find that bone marrow UBC-GFP donor progenitor cells successfully engraft and mature into Ter119+ erythroblast precursors in the marrow and spleen of recipient mice (Figure 6E, supplemental Figure 4B). Furthermore, lineage-depleted progenitors from peripheral blood donors robustly engraft and mature into erythroid precursors in recipient spleen (Figure 6E, supplemental Figure 4B), directly illustrating that circulating progenitors at 6 days after TBI can colonize the spleen. Intriguingly, unlike bone marrow progenitor donors that engraft both marrow and spleen, circulating erythroid progenitors engraft only the spleen and not the marrow of recipient mice (Figure 6E, supplemental Figure 4B). Taken together, these data support the concept that after repopulation of the bone marrow, erythroid progenitors enter the peripheral bloodstream and reseed the spleen.
Discussion
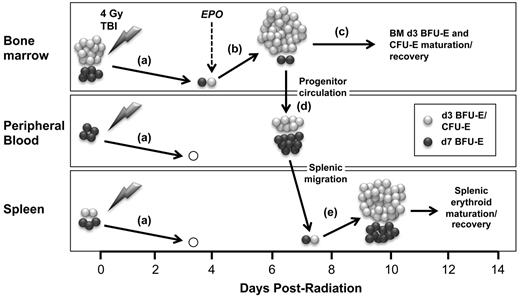
Sublethal irradiation leads to the rapid depletion of almost all erythroid progenitors and precursors in the bone marrow and the spleen (Figure 7A). Thus, sublethal radiation stress, in which the extravascular erythron is depleted but peripheral RBC remain relatively intact, is very different from stress erythropoiesis induced by the acute peripheral anemia after bleeding or hemolysis, in which the circulating RBC compartment is severely injured but the extravascular erythron is preserved. Here, we investigated the cellular kinetics of erythroid recovery, systematically analyzing the progenitor, precursor, and peripheral blood cell compartments of the erythron. We find that sublethal radiation injury leads to a marked reduction in reticulocyte output and the subsequent onset of anemia, which provides the stimulus for the rise in endogenous EPO levels. As demonstrated by physiologic loss-of-function transfusion studies and gain-of-function EPO administration, EPO is both necessary and sufficient to drive the robust expansion of late-stage erythroid progenitors, specifically d3 BFU-E and CFU-E, in the bone marrow (Figure 7B). Administration of EPO at 1 hour after radiation accelerates recovery of late-stage erythroid progenitors in the spleen as well as the bone marrow (supplemental Figure 5). These findings build on previous studies by others suggesting that erythroid recovery from sublethal irradiation initiates in the EPO-responsive compartment6,20-22 and provide direct evidence, for the first time, that this recovery initiates from bone marrow d3 BFU-E.
Model for EPO-induced endogenous recovery of the erythron after sublethal radiation stress centered on the expansion, maturation, and migration of bone marrow erythroid progenitors. (A) Sublethal (4 Gy) irradiation causes the near-total loss of erythroid progenitors and precursors in bone marrow, peripheral blood, and spleen leading to the gradual onset of anemia. (B) This anemia induces EPO, which causes the specific, rapid expansion of late-stage erythroid progenitors (d3 BFU-E and CFU-E) in the bone marrow. (C) d3 BFU-E and CFU-E mature into erythroblast precursors and circulating red cells to provide a rapid, short-term erythroid response to acute radiation stress. (D) Simultaneously, early and late-stage erythroid progenitors transiently circulate into the bloodstream. (E) The spleen is subsequently seeded by circulating erythroid progenitors and undergoes robust erythroid reconstitution to augment recovery of the erythron.
Model for EPO-induced endogenous recovery of the erythron after sublethal radiation stress centered on the expansion, maturation, and migration of bone marrow erythroid progenitors. (A) Sublethal (4 Gy) irradiation causes the near-total loss of erythroid progenitors and precursors in bone marrow, peripheral blood, and spleen leading to the gradual onset of anemia. (B) This anemia induces EPO, which causes the specific, rapid expansion of late-stage erythroid progenitors (d3 BFU-E and CFU-E) in the bone marrow. (C) d3 BFU-E and CFU-E mature into erythroblast precursors and circulating red cells to provide a rapid, short-term erythroid response to acute radiation stress. (D) Simultaneously, early and late-stage erythroid progenitors transiently circulate into the bloodstream. (E) The spleen is subsequently seeded by circulating erythroid progenitors and undergoes robust erythroid reconstitution to augment recovery of the erythron.
Acute anemic stress causes rapid increases in peripheral EPO levels, driving the restoration of the peripheral RBC mass by preventing apoptosis of reserve splenic erythroid progenitors,18,19 by BMP4-mediated expansion of splenic erythroid stress progenitors,14-17 and by self-renewal of ProE-like erythroid precursors.34 In contrast, after sublethal radiation injury, we find that EPO induction leads to the rapid and specific expansion of late-stage erythroid progenitors in the marrow without initial splenic involvement. Furthermore, we find that EPO administration at 1 hour after radiation is unable to rescue apoptotic loss of CFU-E and ProE by 6 hours after TBI (data not shown). These data support a model of direct EPO-mediated expansion of surviving erythroid progenitors in the bone marrow. The robust expansion of late-stage erythroid progenitors may be due, in part, to more rapid differentiation of d7 BFU-E. In support of this mechanism, we find that d7 BFU-E–derived colonies from irradiated mice contain approximately one-half the number of cells found in d7 BFU-E–derived colonies from unirradiated control mice (data not shown). Alternatively, this expansion may be a result of limited self-renewal of late-stage erythroid progenitors. Glucocorticoid signaling is essential for stress erythropoiesis41 and the ex vivo self-renewal of immature erythroblasts.42,43 Recent studies have also implicated glucocorticoid signaling in the regulation of limited BFU-E self-renewal.44 Although we did not detect changes in serum cortisol levels post–4 Gy TBI (data not shown), it is possible that EPO may synergize with endogenous glucocorticoid signaling pathways and other signaling mechanisms to induce rapid expansion and limited self-renewal of d3 BFU-E and CFU-E during recovery from sublethal irradiation.
After the initial expansion of d3 BFU-E and CFU-E at 4 to 6 days after radiation, the EPO-responsive compartment in the bone marrow is sharply down-regulated. This loss of erythroid progenitors in the marrow coincides temporally with a wave of maturing erythroid precursors (Figure 7C). The EPO-mediated expansion of late-stage erythroid progenitors and their maturation into downstream precursors provides a bone marrow-derived and lineage-specific mechanism of rapid erythroid recovery in times of acute stress. Unlike the largely splenic response after peripheral anemic stress, this marrow-centered response to radiation injury in the mouse more closely resembles the human stress erythropoietic response, which occurs in the bone marrow. Sublethal irradiation injury thus provides a novel model for the study of the endogenous recovery of the erythroid lineage in the bone marrow. Furthermore, we anticipate that this in vivo model will serve to evaluate potential therapeutic factors that regulate or modulate erythroid cell maturation.
In addition to rapid maturation of erythroid progenitors in the marrow, the down-regulation of erythroid progenitors at 6 to 7 days after radiation is also temporally associated with erythroid progenitor emergence into the bloodstream (Figure 7D). Although some BFU-E normally circulate, we found the transient emergence in the bloodstream not only of BFU-E, but also CFU-E, as well as all stages of erythroid precursors. Interestingly, circulating erythroid progenitors have significantly decreased levels of surface integrins, particularly α4-integrin, which plays an important role in the adherence of erythroid precursors to macrophage cells within erythroblast islands.35 Our data raise the possibility that changes in integrin expression may play a role in the emergence of erythroid progenitors into the bloodstream by allowing for greater detachment and mobility of erythroid progenitors from the marrow microenvironment. Evidence exists for the increased circulation of BFU-E in the bloodstream after phenylhydrazine stress.45 In addition, transplanted short-term hematopoietic stem cells have been shown to home to the spleen of lethally irradiated mice and form stress erythroid progenitors.16,17 However, endogenous erythroid progenitor migration from bone marrow to spleen has not been previously demonstrated after acute erythroid stress (Figure 7D-E). In addition, the selective splenic engraftment by these circulating erythroid progenitors in our short-term transplantation studies supports the concept that they are responsible for re-initiation of extramedullary erythropoiesis after radiation injury (Figure 7E).
We find that re-initiation and expansion of the splenic erythron occurs only after reconstitution and filling of the marrow space and subsequent emergence of erythroid progenitors into the bloodstream at 6 days after TBI. These findings support the concept that extramedullary erythropoiesis in murine spleen occurs only after the marrow is unable to meet the synthetic demand for RBCs,46 and suggest that splenic erythropoiesis may be necessary to compensate for the lack of sufficient marrow space in rodents. In contrast, erythropoietic activity does not normally migrate to extramedullary sites after acute anemic stress in humans. This difference may potentially be because of greater capacity for erythroid expansion in the human marrow. However, chronic severe pathologic stressors such as myelofibrosis, thalassemia, or sickle cell anemia leads to extramedullary erythropoiesis in humans.47-51 The mechanisms regulating the establishment of extramedullary erythropoiesis have yet to be elucidated. Thus, the re-establishment of splenic erythropoiesis during recovery from sublethal radiation provides a model for the future investigation of erythroid progenitor circulation and homing to extramedullary sites.
We conclude that sublethal radiation, which is characterized by specific injury to the extravascular erythron, initial expansion and maturation of EPO-responsive erythroid progenitors exclusively in the marrow, and subsequent reseeding of extramedullary sites, serves as a novel model of endogenous stress erythropoiesis (Figure 7). Despite significant differences in the recovery processes after sublethal radiation stress and peripheral anemic stress, both processes are dependent on feedback from peripheral blood to late-stage erythroid progenitors in the marrow mediated by EPO, indicating that EPO is the central regulator of recovery of the erythron after acute stress. Granulocyte colony stimulating factor (G-CSF) and granulocyte-monocyte stimulating factor (GM-CSF) are currently stockpiled for treatment of acute hematopoietic syndrome in humans.52,53 In addition, animal studies indicate that cytokine therapies directed toward multipotential progenitors improve hematopoietic recovery after radiation injury and suggest that combination cytokine therapies may be most effective in treatment of radiation-induced cytopenias.54-56 EPO therapy has also been shown to improve survival after lethal radiation exposure23 and our data indicate that exogenous EPO can accelerate recovery of the erythron. These data raise the possibility that EPO, in combination with other cytokines, may play a useful role in treatment of the acute hematopoietic syndrome after accidental or intentional radiation exposure.
The online version of this article contains a data supplement.
The publication costs of this article were defrayed in part by page charge payment. Therefore, and solely to indicate this fact, this article is hereby marked “advertisement” in accordance with 18 USC section 1734.
Acknowledgments
The authors thank Dr Ben Frisch for his assistance with transplant experiments, and Sarah Mack and Jenny McLaughlin for their assistance with immunohistochemistry. They also acknowledge Dr Tim Bushnell and the University of Rochester Medical Center Flow Core for their invaluable assistance and support of this project.
This work was supported by funding from National Institute of Allergy and Infectious Diseases (NIAID) R01 AI080401 (J.P.), National Institute of Diabetes and Digestive and Kidney Diseases F30 DK085706 (S.A.P.), NIAID Center for Medical Countermeasures against Radiation Program (U19 AI067733 pilot program and U19 AI091036-1, J.P.), and the Michael Napoleone Foundation. S.A.P. is a trainee in the Medical Scientist Training Program funded by National Institutes of Health T32GM07356.
National Institutes of Health
Authorship
Contribution: S.A.P. designed and performed experiments, analyzed data, and wrote the paper; J.W., P.D.K., A.D.K., and K.E.M. designed and performed experiments; J.C.B. performed experiments and analyzed data; and J.P. designed experiments, analyzed data, and wrote the paper.
Conflict-of-interest disclosure: J.C.B. is an employee of Litron Laboratories, which is pursuing patent protection for methods and products to assess hematotoxicity resulting from exposure to clastogenic injury via enumeration of blood cells using flow cytometry. The remaining authors declare no competing financial interests.
Correspondence: James Palis, Center for Pediatric Biomedical Research, University of Rochester Medical Center, Box 703, 601 Elmwood Ave, Rochester, NY, 14642; e-mail: james_palis@urmc.rochester.edu.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal